MRM Insights: How gut stem cells are joining the fight against COVID-19

Dr. Alexander Gregorieff
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Alexander Gregorieff, Assistant Professor in the Department of Pathology at the RI-MUCH and member of the MRM Executive Committee member, discusses how gut stem cells are joining the fight against COVID-19.
How gut stem cells are joining the fight against COVID-19
COVID-19 has emerged in recent months as the most pressing public health and social issue throughout the world. Although current estimates of the fatality rates remain disputed, the disease caused by SARS-CoV-2 ranges from mild flu-like symptoms to acute respiratory distress and multi-organ failure. Accumulating evidence suggests that hyper-inflammatory responses drive widespread tissue damage observed in severe COVID-19 cases 1. However, the reasons why certain individuals develop exacerbated immune responses in the face of this novel coronavirus remain uncertain. A research team led by Dr. Irah King of the RI-MUHC and recently funded by McGill Interdisciplinary Initiative in Infection and Immunity (MI4), propose that the microbes living in our guts may be crucial determinants driving the course of COVID-19.
The case for gut microbes.
Although best characterized by acute and progressive respiratory complications, several reports now indicate that COVID-19 is closely associated with gastrointestinal (GI) symptoms including nausea and diarrhea 2,3. Importantly, the presence of SARS-Cov-2 has been found in both stool samples and gut mucosal biopsies of infected individuals even in the absence of positive lung samples 4. Although no evidence of widespread intestinal tissue damage has been reported in COVID-19 patients, these early findings implicate the gut as possibly a key component modulating disease severity.
The gut microbiota (a collection of bacteria, viruses, fungi and archaea) is critical for efficient absorption of nutrients and energy metabolism, but also sets the inflammatory “tone” of extra-intestinal organs 5. As a result, it has been established that the gut microbiota has potent regulatory functions during pulmonary viral infection 6. Conversely, enhancing the diversity of the gut microbiota results in decreased morbidity and mortality following a lethal influenza challenge 7. Consistent with these studies, previous collaborative work by Dr. King’s team and the laboratories of Drs. Maziar Divangahi and Jeff Xia from McGill University have shown that modulation of the gut microbiota has striking effects on host resistance to Mycobacterium tuberculosis infection 8. Based on these findings, these researchers are now turning their attention to addressing whether a similar connection may exist between gut microbiota and host immune responses to SARS-CoV-2.
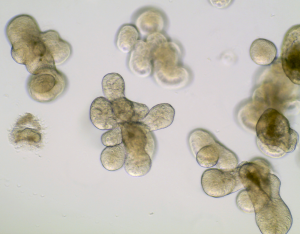
Photo Credit: Dr. Tanvi Anil Javkar
Modeling COVID-19 in a dish.
As a first step to unlocking this mystery, King and colleagues wish to investigate whether SARS-CoV-2 infection affects the innate anti-microbial response of the gut epithelium. Indeed, the gut epithelial lining serves as formidable barrier against pathogens through its potent arsenal of secreted factors that neutralize potential bacterial invaders. Along these lines, a previous report has shown that the receptor for SARS-CoV-2 entry into the cell (i.e. ACE-2) is abundantly expressed on epithelial cells of the GI tract and loss of this receptor in mice is linked to microbial dysbiosis and defective regenerative responses in the gut 9. As a means to address this question, Dr. King’s team, in collaboration with Dr. Alex Gregorieff (RI-MUHC), will develop a human intestinal organoid system to model SARS-CoV-2 infection in the gut.
Organoids are near-physiological cell culture systems maintained by tissue specific stem cells in a 3D microenvironment that exhibit similar organ functionality as their tissue of origin 10. Organoids have been widely used to shed light on adult stem cell behaviour in both health and disease, as well as study how common pathogens such as Norovirus, Helicobacter pylori and even enteric parasites interact with the gut epithelium 11–13. More recently, there has been a race to exploit lung, endothelial, kidney and liver organoids to study the impact of SARS-CoV-2 on these tissues 14–16. Importantly, organoids have also proven to be powerful models to test novel therapies aimed at blocking the ability of SARS-CoV-2 to infect cells 15. The research team from the RI-MUHC anticipates that their studies using stem cell-based organoids will reveal important insight into this devastating disease and help determine whether the intestinal ecosystem should be targeted as part of a comprehensive treatment plan to fight the COVID-19 pandemic.
References:
1. Blanco-Melo, D. et al. Imbalanced hostresponse to SARS-CoV-2 drives development of COVID-19. Cell (2020). doi:DOI: 10.1016/j.cell.2020.04.026
2. Gu, J., Han, B. & Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology (2020). doi:10.1053/j.gastro.2020.02.054
3. Pan, L. et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. gastrology Pre-proof (2020).
4. Xiao, F. et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology (2020). doi:10.1053/j.gastro.2020.02.055
5. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–14 (2012).
6. Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 108, 5354–9 (2011).
7. Rosshart, S. P. et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 171, 1015-1028.e13 (2017).
8. Khan, N. et al. Intestinal dysbiosis compromises alveolar macrophage immunity to Mycobacterium tuberculosis. Mucosal Immunol. 12, 772–783 (2019).
9. Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–81 (2012).
10. Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–54 (2016).
11. Wilke, G. et al. A Stem-Cell-Derived Platform Enables Complete Cryptosporidium Development In Vitro and Genetic Tractability. Cell Host Microbe 26, 123-134.e8 (2019).
12. Ramani, S., Crawford, S. E., Blutt, S. E. & Estes, M. K. Human organoid cultures: transformative new tools for human virus studies. Curr. Opin. Virol. 29, 79–86 (2018).
13. Pompaiah, M. & Bartfeld, S. Gastric Organoids: An Emerging Model System to Study Helicobacter pylori Pathogenesis. Curr. Top. Microbiol. Immunol. 400, 149–168
14. Zhu, N. et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020).
15. Monteil, V. et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell (2020). doi:DOI: 10.1016/j.cell.2020.04.004
16. Zhao, B. et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell (2020). doi:10.1007/s13238-020-00718-6
