
MRM Insights: Is there a role for cell therapy in SARS-CoV-2 infection?

Dr. Michel L. Tremblay

Dr. Inés Colmegna
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Inés Colmegna, Associate Professor in the Division of Rheumatology at the RI-MUCH and member of the MRM Executive Committee member, and Dr. Michel L. Tremblay, Professor in the Department of Biochemistry at the Goodman Cancer Research Centre and Director of the MRM Network ask if there is a role for cell therapy in SARS-CoV-2 infection.
Is there a role for cell therapy in SARS-CoV-2 infection?
The impact of the current pandemia is unprecedented and has changed people, societies, and the world at large. There is an urgent need for approaches that proven valid can alter the natural course of the infection. Is there as role for cell therapy in this paradigm?
Clinically, COVID-19 illness can be sub-divided into three phases of increasing severity1 (Fig.1). Stage I, characterized by early infection with mild symptoms; Stage II, defined by pulmonary involvement without (IIa) or with (IIb) hypoxia; and Stage III (critical), marked by systemic hyperinflammation1. Consistently throughout studies, ~80% of cases are mild, 14% severe, and 5% critical. In critical cases, the fatality rate approaches 50%2.
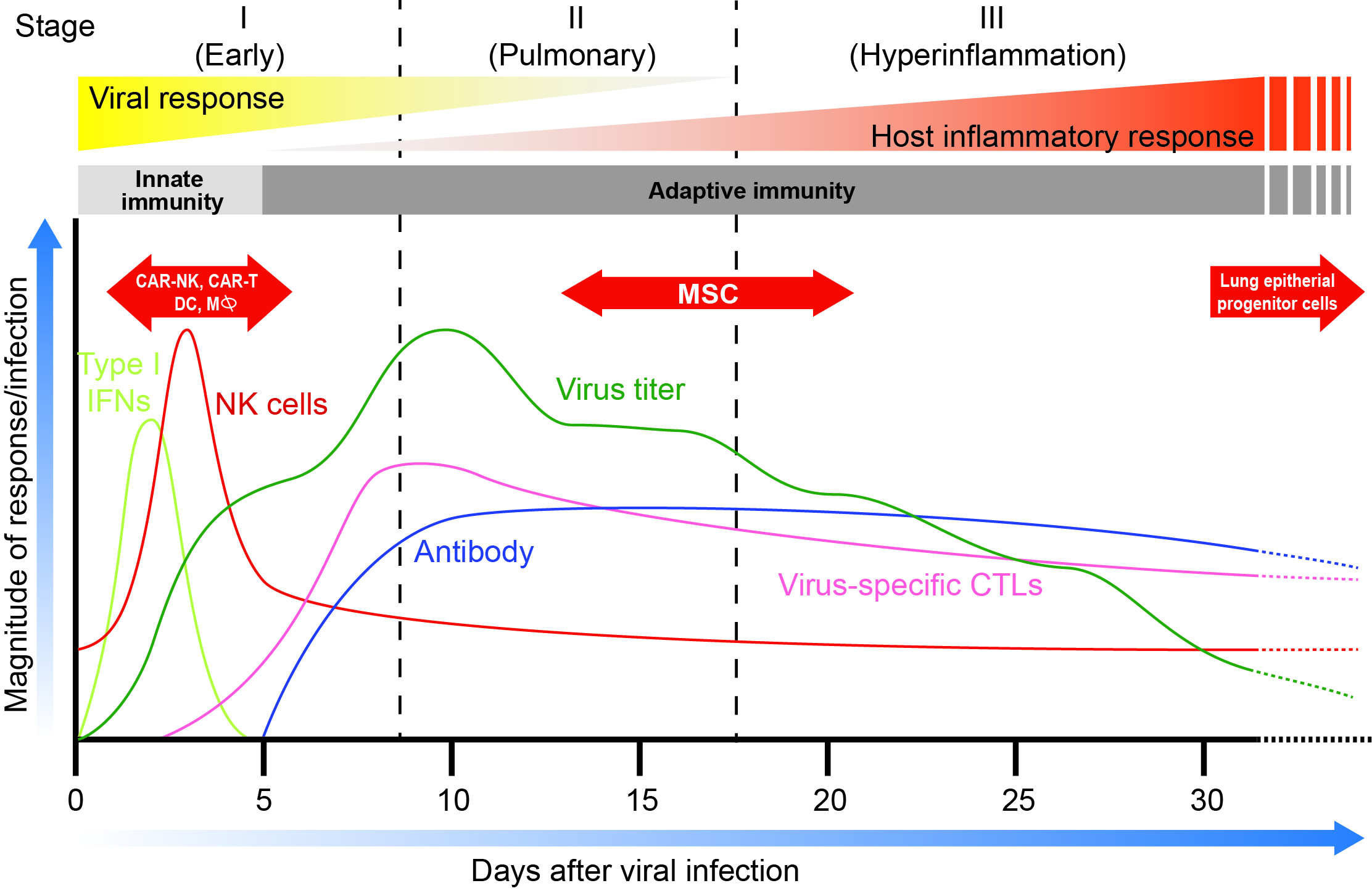
Figure 1.Time dependent profile of the main stages of COVID’s patient’s infection with corresponding patient’s immune cells and ANTI-COVID antibodies levels. In red are the optimal periods of cell therapy treatments for various cell immunotherapies. Adapted from Siddiqi et al. (REF.1). Note that “Virus titer” levels are based on a recent paper by Carmo et al. J. Med. Virol. June 2, 2020 (REF 26)
Pathogenically, the host response is a key determinant of COVID-19 outcomes. Increased levels of IL-6, Ferritin, D-dimer, Toponin I and reduced lymphocyte counts predict mortality3. These features are shared by the ‘cytokine storm syndromes’, a group of conditions in which hyperinflammation and multiorgan disease result from the excessive cytokine release from uncontrolled immune activation4. This provides the rationale for ongoing clinical trials assessing the safety and efficacy of cytokine blockade strategies including the modulation of IL-1, IL-6, and IFNγ and others (Table 1). An alternative approach currently investigated for cytokine storm syndromes is the systemic administration of multipotent mesenchymal stromal cells (MSCs)5,6.

Table 1: COVID current and potential therapies. Source: Dr. Inés Colmegna and Dr. Michel L. Tremblay
MSCs are multipotent progenitor cells with immunomodulatory, pro-angiogenic, and anti-fibrotic properties7. The most common sources of MSCs used in clinical trials are the bone marrow (BMMSC), adipose tissue (ATMSC) and umbilical cord (UCMSC). In Canada, the use of BMMSCs is approved for pediatric refractory acute graft versus host disease8. MSCs show intermediate levels of human leukocyte antigen (HLA) class I molecules and have no detectable levels of HLA class II, including HLA-DR, and co-stimulatory molecules (CD40, CD80, and CD86)9. This phenotype allows their allogeneic use8. In animal models, after intravenous administration, most UCMSC are trapped in the lungs and are rapidly removed from the circulation (half-life of ~ 24 hours)10. This is particularly relevant as lungs are the primary target organ of COVID-19. In the lungs, infused UCMSC are rapidly phagocytosed by classical monocytes and tissue resident alveolar macrophages that polarize towards an M2 anti-inflammatory phenotype (e.g. express programmed death ligand-1 and IL-10)11. UCMSC-primed monocytes induce regulatory T cell formation amplifying and prolonging the anti-inflammatory effects of MSCs10. Another mechanism involved in the anti-inflammatory and trophic roles (e.g., inhibition of apoptosis, angiogenesis) of MSCs is the secretion of growth factors, cytokines, and hormones (e.g., VEGF, PDGF, ANG-1, IL-11, PGE2, TSG-6, SDF-1, HGF, IGF-1, IDO), membrane particles, mitochondria and extracellular vesicles12-14. The production of these mediators is enhanced post-MSC priming by pro-inflammatory cytokines including interferon-γ (IFN-γ), TNF-α, IL-17, and IL-1β15. As indicated earlier, these factors are elevated in patients with COVID-19 and thus, could prime UCMSC upon infusion enhancing their therapeutic effects. This provides the rationale for over 20 MSC’s based clinical trials for COVID-19 that are registered in ClinicalTrials.gov. Previous evidence in other disease settings and limited data specifically in COVID-19 supports the safety of this approach16,17,18. Four randomized, placebo-controlled, Phase 2/3 trials will address the role of MSCs as therapy for COVID. Table 2 summarizes the key features and primary outcomes of those trials.
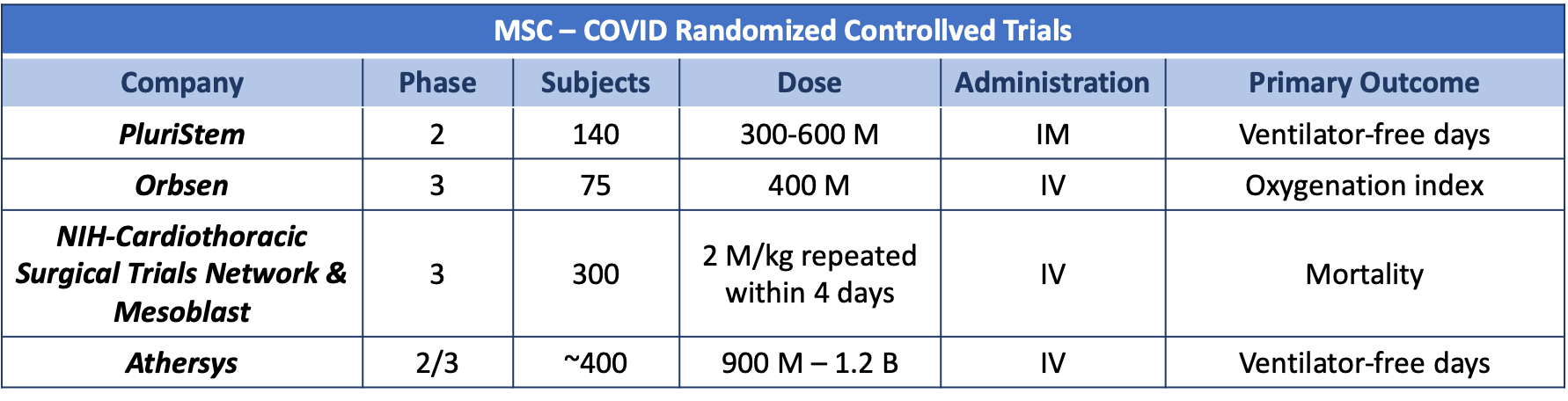
Table 2: M: million; B: billion; IM: intramuscular, IV: intravenous Sources: Dr. Inés Colmegna and ClinicalTrials.gov
In addition to MSCs, other cellular therapeutic approaches could potentially be used in COVID-19 patients at high-risk for adverse outcomes. Among those, immune cell transfer such as CAR-T already employed in cancer 19 and other infectious diseases 20 could be used to directly eliminate viral infected cells or to improve the innate early immune response before the severity of SARS-CoV-2 infection becomes overwhelming. For example, autologous cell infusion of exogenously activated dendritic cells or even M1 activated macrophages presenting specific coronavirus antigens may help the more vulnerable patients to reduce their viral load. Similarly, chimeric associated receptor modified natural killer cells (CAR-NK) may be used for targeting infected cells. It is important to consider that such cell therapy approaches should be attempted early in the infection; as once a severe inflammatory response is established (phase II) activated (genetically modified or not) immune cells may lead to more severe outcomes. Another stage at which cell therapy may also be beneficial in COVID-19 is for individuals that recovered from infection (post-phase III) and have permanent lung epithelial fibrotic damage 21. Upon discharge, >90% of COVID patients have residual lung disease detected by CT 22. Some authors proposed post-infection, to use antifibrotic therapies to minimize long term lung damage 23. Alternatively, for patients more severely affected, the transfer of lung epithelial progenitor cells may be a potential therapeutic option that requires to be tested in clinical trials 24. This approach has been also proposed for both influenza and coronaviruses 25.
Is there a role for cell therapy in SARS-CoV-2 infection? Based on the current understanding of COVID-9 pathogenesis and prior mechanistic knowledge of cell therapy approaches, it is tempting to speculate that it might be. However, it is only through well-designed clinical trials that the efficacy of these approaches for COVID should be tested. Those studies will benefit from being associated with mechanistic data in this specific disease model and for having specific patient selection criteria. As in every other condition, it is unrealistic to expect a ‘one size fits all’ type of solution. In contrast, applying previous knowledge and rigorously testing it in this novel disease will allow identifying solutions to alleviate the burden of the pandemia and promote the recovery of affected patients.
References:
1. Siddiqi HKM, M.R. COVID-19 illness in native and immunosuppressed states: A clinical−therapeutic staging proposal. The Journal of Heart and Lung Transplantation 2020; 39(5): 405-7.
2. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020.
3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229): 1054-62.
4. Henderson LA, Canna SW, Schulert GS, et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol 2020.
5. Swart JF, de Roock S, Nievelstein RAJ, Slaper-Cortenbach ICM, Boelens JJ, Wulffraat NM. Bone-marrow derived mesenchymal stromal cells infusion in therapy refractory juvenile idiopathic arthritis patients. Rheumatology (Oxford) 2019; 58(10): 1812-7.
6. Saldana L, Bensiamar F, Valles G, Mancebo FJ, Garcia-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther 2019; 10(1): 58.
7. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 2016; 7(1): 125.
8. Galipeau J, Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018; 22(6): 824-33.
9. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003; 31(10): 890-6.
10. de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018; 36(4): 602-15.
11. de Witte SFH, Merino AM, Franquesa M, et al. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res Ther 2017; 8(1): 140.
12. Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol 2018; 9: 2837.
13. Hoogduijn MJ, Lombardo E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl Med 2019; 8(11): 1126-34.
14. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med 2017; 196(10): 1275-86.
15. Noronha NC, Mizukami A, Caliari-Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther 2019; 10(1): 131.
16. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 2020; 11(2): 216-28.
17. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 2019; 7(2): 154-62.
18. Schijns V, Lavelle EC. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur J Immunol 2020.
19. David Pettitt 1, Zeeshaan Arshad 2, James Smith 3, Tijana Stanic 4, Georg Holländer 5, David Brindley . CAR-T Cells: A Systematic Review and Mixed Methods Analysis of the Clinical Trial Landscape. Mol Ther. Feb 7;26(2):342-353. 2018 doi: 10.1016/j.ymthe.2017.10.019. Epub 2017 Nov 2.
20. Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation .J Hematol Oncol. Feb 6;12(1):13. 2019 doi: 10.1186/s13045-019-0701-1.PMID: 30728058.
21. Paolo Spagnolo, Elisabetta Balestro, Stefano Aliberti, Elisabetta Cocconcelli, Davide Biondini, Giovanni Della Casa, Nicola Sverzellati, Toby M Maher Pulmonary Fibrosis Secondary to COVID-19: A Call to Arms? Lancet Respir Med 2020 Published Online May 15, 2020 https://doi.org/10.1016/ S2213-2600(20)30222-8
22. Yuhui Wang*, Chengjun Dong, Yue Hu, Chungao Li, Qianqian Ren, Xin Zhang, Heshui Shi, Min Zhou Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. RADIOLOGY Published Online:Mar 19 2020 https://doi.org/10.1148/radiol.2020200843
23. Peter M George, Athol U Wells, R Gisli Jenkins Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy Lancet Respir Med 2020 PublishedOnline May 15, 2020 https://doi.org/10.1016/ S2213-2600(20)30225-3
24. William J. Zacharias, David B. Frank, Jarod A. Zepp, Michael P. Morley, Farrah A . Alkhaleel, Jun Kong, Su Zhou, Edward Cantu and Edward E. Morrisey. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 8 m a r c h VO L 5 5 5 | | 2 5 1-273.
25. Jiang Du , Han Li , Jie Lian , Xinxing Zhu , Liang Qiao and Juntang Lin. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury Stem Cell Research & Therapy (2020) 11:192 https://doi.org/10.1186/s13287-020-01699-3
