MRM Insights – The long and winding road: Human germline genome editing

Me. Erika Kleiderman
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Me. Erika Kleiderman, Academic Associate at the Centre of Genomics and Policy, member of the MRM Executive Committee and Chair of the MRM Ethics Committee, talks about the long and winding road of human germline genome editing.
The long and winding road: Human germline genome editing
Human genome editing brings with it the potential to edit or modify human DNA so as to treat (and eventually even prevent) various genetic diseases or conditions (NASEM 2017). When discussing human genome editing, there is an important distinction to be made between somatic editing (i.e., modifications are limited to the treated individual) and germline editing (i.e., heritable modifications that can potentially be passed on to future generations), which will be the focus here. Somatic editing is deemed ethically acceptable and is regulated under existing guidance related to cell and gene therapies; whereas germline editing is deemed to be both morally and ethically contentious, and generally prohibited (i.e., should not be used in a clinical context). See Figure 1 for a summary of key distinctions between the two.
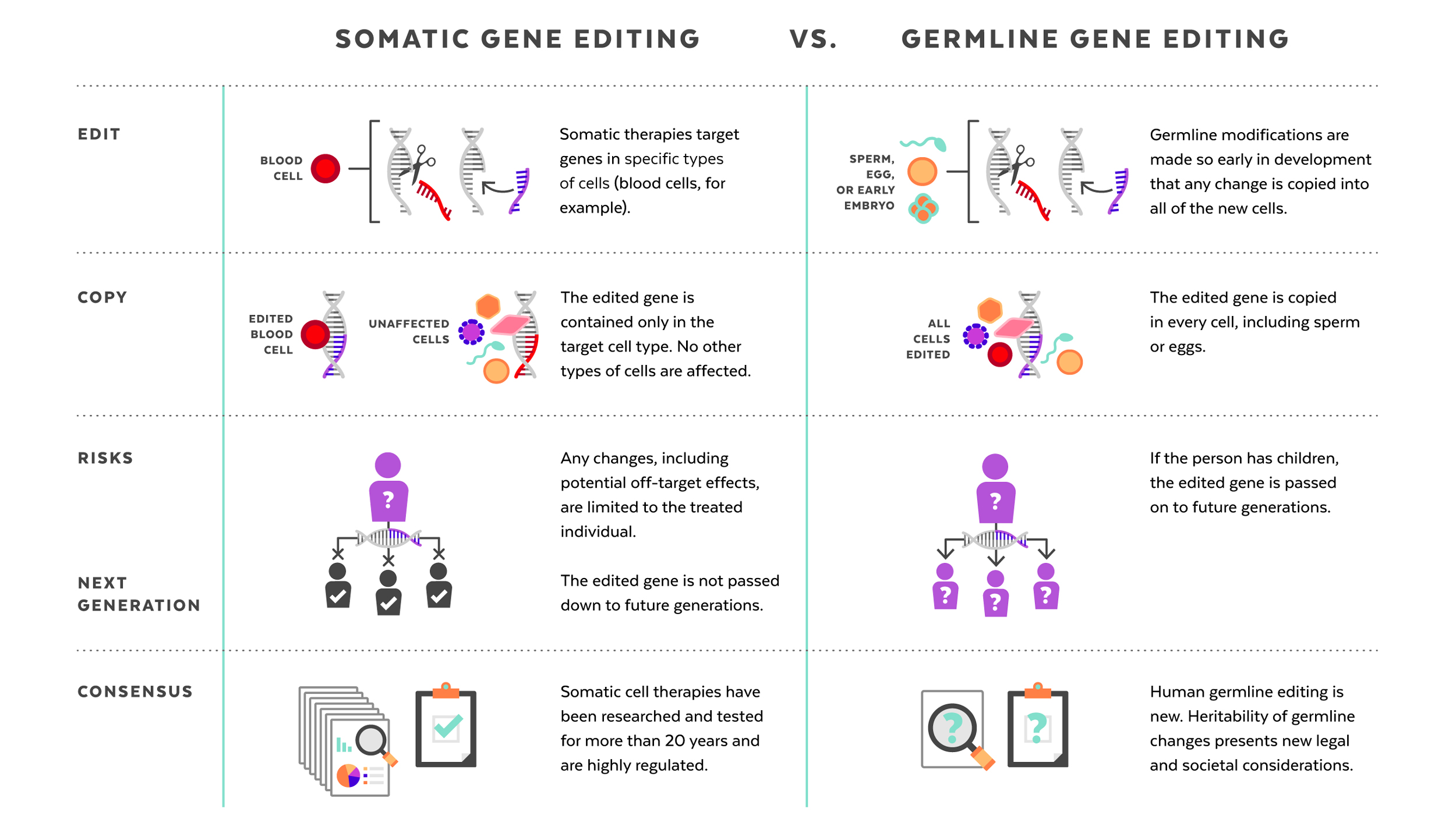
Figure 1. Summary key distinctions between somatic and germline genome editing. From the Harvard Gazette, Perspectives on gene editing.
Human germline genome editing (HGGE) has been at the forefront of both national and international ethical debates since the advent of CRISPR-Cas91 in 2015. Since 2015, more than 61 ethics / policy statements and reports have been published by more than 50 countries and organizations (Brokowski 2018). Most of these statements, in alignment with national laws and regulations, encourage somatic genome editing, while prohibiting the use of HGGE for reproductive purposes (Baylis et al. 2020; Isasi et al. 2016). Moreover, the call for broad societal discussion (and hopefully eventual consensus) continues to gain importance as these discussions shift from the policymaking realm to incorporate a broader range of stakeholders and end users. The goal being to expand the scope of perspectives and experiences beyond scientists, clinicians, ethicists and policymakers to include those who could or would be most directly affected by advancements and applications of this technology.
In order to trace the evolution of the germline debate on both the science and policy fronts, I provide a high-level overview of key events and reports shaping the debate since 2015.
April 2015:
The publication of a Chinese study (Liang et al. 2015) that used HGGE on non-viable human embryos in an attempt to treat β-thalassemia. Although the study was not itself very successful, it served as the catalyst for the international debate surrounding CRISPR-Cas9 and its application in human embryos.
December 2015:
The first International Summit on Human Genome Modification was held in Washington, D.C. to discuss the implications of using HGGE in human embryos. Ultimately, it was determined that “it would be irresponsible to proceed with any clinical use of germline editing unless and until (i) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (ii) there is broad societal consensus about the appropriateness of the proposed application” (Baltimore et al. 2015). Therefore, the application of HGGE for reproductive purposes should not be permitted at this time.
February 2016:
The Human Fertilisation and Embryology Authority (UK) granted its first research license to Dr. Kathy Niakan permitting the use of genome editing on healthy human embryos within a research context (Callaway 2016). The purpose of the research was to better understand non-implantation and miscarriage. All embryos used for this research were destroyed in accordance with the internationally accepted 14-day rule (Pera 2017). Similar research has also been conducted by Dr. Fredrik Lanner in Sweden with the aim of developing a deeper understanding of infertility, miscarriages, and human embryonic stem cells (Gallego 2016).
February 2017:
The publication of the National Academies of Sciences, Engineering and Medicine (NASEM) report entitled Human Genome Editing – Science, Ethics & Governance. First, this report states that genome editing for research purposes should be permitted, as this is captured by existing regulations. Second, the use of somatic genome editing in the context of clinical trials should be permitted, as existing regulations and norms for cell and gene therapies can be applied to ensure proper oversight. Third, the use of germline genome editing in the context of clinical trials may be permitted only for the treatment and prevention of serious diseases (e.g., compelling purposes), provided that such trials meet a set of stringent criteria (e.g., last resort, long-term multigenerational follow-up, continued public engagement) and a rigorous oversight system is in place. Fourth, the use of human genome editing for purposes other than the treatment and prevention of disease (i.e., human enhancement) should remain banned at this time. Therefore, this report is the first to suggest the possibility that clinical applications of HGGE may eventually be permissible under certain circumstances and conditions.
August 2017:
The publication of a U.S. study, led by Dr. Shoukhrat Mitalipov, that used HGGE in an attempt to correct a serious, heritable heart condition (Servick 2017; Ma et al. 2017). This study was conducted using healthy human embryos that were destroyed in accordance with the 14-day rule. As the most “successful” use of the technology to correct targeted genes in human embryos to date, it showcased the potential to repair mutations and prevent reproductive transmission of heritable diseases. This garnered much attention and excitement in the media, while also furthering our understanding of some of the safety and efficacy issues surrounding HGGE. That being said, these findings are but one step on the long and winding road ahead.
July 2018:
The publication of the Nuffield Council on Bioethics’ report entitled Genome Editing and Human Reproduction – Social and Ethical Issues. This report states that genome editing is not unacceptable in itself, and that it may become morally acceptable in time. Nevertheless, the report stipulates that should HGGE be permitted, it ought to be strictly regulated (e.g., by a regulatory body) and it should be licensed on a case-by-case basis. The permissibility of its use in the context of clinical trials should be limited to studies that have developed a plan for the long-term monitoring of effects on individuals and groups. Finally, the report also encourages broad and inclusive societal debate on HGGE interventions and related scientific and medical developments. In this sense, it begins to draft a possible responsible path forward for HGGE with clear and additional guidelines.
November 2018:
The announcement of the birth of the “CRISPR babies” (twin girls named Lulu and Nana) via a YouTube video was made only days before the second International Summit on Human Genome Modification (Kolata, Wee, and Belluck 2018). Dr. Jiankui He, a Chinese biophysics researcher, genetically modified human embryos and then implanted them into a woman for reproductive purposes. As we have seen, international consensus states that reproductive applications of HGGE should remain banned from both an ethical and scientific standpoint. Not only was the use of HGGE for reproductive purposes premature and irresponsible, but Dr. He’s clinical trial had many shortcomings from a research ethics perspective to say nothing of the potential violations of both Chinese regulations and internationally accepted standards (Krimsky 2019; Barrangou 2018). These shortcomings have direct implications for the integrity of the study itself and for the safety of the participants. This event has led to renewed calls for a moratorium on HGGE in an attempt to ensure that a similar situation does not reoccur (Lander et al. 2019; Adelman et al 2019). Yet, this would be paradoxical, as legal prohibitions surrounding HGGE already exist, so a more realistic route would be to focus on reinforcing current laws, regulations and guidelines, while encouraging participative public dialogue (Knoppers & Kleiderman 2019). Although Dr. He has been sentenced to three years in prison along with a sizable fine, his case has ultimately been reduced to one of illegal medical practice (i.e., practice of medicine without a license), as well as a ban from further research in the area of assisted reproductive technologies (Cohen & Normille 2020; Johnston 2019). Finally, the “CRISPR babies” have also led to discussions about how to prevent rogue scientists and what mechanisms could have encouraged those who knew of Dr. He’s plans to denounce him (Charo 2019; Kofler 2019; Townsend 2020).
November 2018:
The second International Summit on Human Genome Modification was held in Hong Kong to discuss ethical and cultural perspectives, the potential risks and benefits of human genome editing, regulatory / policy considerations, as well as public engagement efforts. The outcomes of the discussion focus on proposing “a translational pathway to germline editing [which] will require adhering to widely accepted standards for clinical research, including criteria articulated in genome editing guidance documents published in the last three years. Such a pathway will require establishing standards for preclinical evidence and accuracy of gene modification, assessment of competency for practitioners of clinical trials, enforceable standards of professional behavior, and strong partnerships with patients and patient advocacy groups” (Baltimore et al. 2018). All the while, human genome editing research should continue to be encouraged so as to foster a better understanding of the safety and efficacy of the technology. Finally, there was an urge to continue encouraging an ongoing international forum / discussion, which has become a common thread throughout the HGGE debate. Yet, the operationalization and logistics of such a forum remain to be determined.
December 2018:
The World Health Organization (WHO) established its Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Gene Editing. The purpose of this Committee is to develop a strong, comprehensive global governance framework for responsible stewardship of human genome editing (Reardon 2019). In addition, the Committee has also called for and established a new global registry for clinical trials using human genome editing (Chaib 2019; Cohen 2019). This registry includes clinical trials using human genome editing on eggs, sperm, and early embryos (germline), as well as on adult cells (somatic). In early 2020, there was an online consultation of the Draft Governance Framework for Human Genome Editing. The final framework is expected to launch in the coming months.
May 2019:
The National Academy of Medicine, the National Academy of Sciences, and the Royal Society established the International Commission on the clinical use of heritable human genome editing. The purpose of this Commission was to “develop a framework for scientists, clinicians, and regulatory authorities to consider when assessing potential clinical applications of human germline genome editing. The framework will identify a number of scientific, medical, and ethical requirements that should be considered, and could inform the development of a potential pathway from research to clinical use – if society concludes that heritable human genome editing applications are acceptable” (NASEM 2019).
June 2019:
A Russian molecular biologist, Dr. Denis Rebrikov, announced his plans to follow in the steps of Dr. He and use HGGE to create HIV-resistant babies, but in a more ethical way than was previously done. Since this announcement, Dr. Rebrikov’s plans have shifted from HIV resistance to editing the embryos of couples with a specific type of inherited deafness so as to create hearing babies. However, the Ministry of Health of the Russian Federation affirmed in October 2019 that the use of HGGE in such a context was premature and that they endorse the WHO position that it would be irresponsible and unacceptable to use genome-edited embryos to initiate human pregnancies at this time (Cyranoski 2019).
November 2020:
The publication of the International Commission on the clinical use of heritable human genome editing’s final report entitled Heritable Human Genome Editing: Consensus Study Report. This report maintains that reproductive applications of HGGE should not proceed until CRISPR’s safety and efficacy have been proven, while calling for continued efforts to establish a broad societal dialogue on the matter. Similar to other reports, the Commission has not ruled out the possibility for potential uses of HGGE; yet these will require further scientific validation via the collection of preclinical data (i.e., encourage research). Moreover, stringently defined criteria and rigorous oversight will also be required of any eventual application, and such applications would need to proceed incrementally. Furthermore, the Commission proposes the establishment of an International Scientific Advisory Panel (ISAP) that would be responsible for overseeing any clinical use of HGGE. Members of the ISAP would possess diverse expertise and experience allowing them to evaluate and provide recommendations based on the proposed applications of HGGE. Finally, they flag the importance of establishing an international mechanism that would enable the transmission or public disclosure of any concerns or uses of the technology outside of the approved contexts (established guidelines and standards) to relevant national authorities.
1CRISPR: Clustered regularly interspaced short palindromic repeats.
References
Adelman B, Albright C, Andrews L., et al. (2019). Letter to the Secretary of the U.S. Department of Health and Human Services. Available from: https://www.asgct.org/global/documents/clinical-germline-gene-editing-letter.pdf.
Baltimore, D., Charo, A., Daley, G. Q., Doudna, J. A., Kato, K., Kim, J-S., et al. (2018). Statement by the Organizing Committee of the Second International Summit on Human Genome Editing. Available from: https://www.nationalacademies.org/news/2018/11/statement-by-the-organizing-committee-of-the-second-international-summit-on-human-genome-editing
Baltimore, D., Baylis, F., Berg, P., Daley, G. Q., Doudna, J. A., Lander, E. S., et al. (2015). On Human Gene Editing: International Summit Statement. Available from: https://www.nationalacademies.org/news/2015/12/on-human-gene-editing-international-summit-statement.
Barrangou, R. (2018). CRISPR Crossroads for Genome Editing. The CRISPR Journal, 1(6), 349–350.
Baylis, F., Darnovsky, M., Hasson, K., & Krahn, T. M. (2020). Human Germline and Heritable Genome Editing: The Global Policy Landscape. The CRISPR Journal, 3(5), 365-377.
Brokowski, C., 2018. Do CRISPR Germline Ethics Statements Cut It? The CRISPR Journal, 1(2), p.115-125.
Callaway, E. (2016). Embryo editing gets green light. Nature, 530, 18.
Chaib, F. (2019). WHO launches global registry on human genome editing. WHO News. Available from: https://www.who.int/news/item/29-08-2019-who-launches-global-registry-on-human-genome-editing.
Charo, A. (2019) Rogues and regulation of germline editing. The New England Journal of Medicine, 380, 976-980.
Cohen, J. (2019). WHO panel proposes new global registry for all CRISPR human experiments. Science. doi: 10.1126/science.aax3948.
Cohen, J., & Normille, D. (2020). China delivers verdict on gene editing of babies. Science, 367(6474), 130.
Cyranoski, D. (2019). Russian ‘CRISPR-baby’ scientist has started editing genes in human eggs with goal of altering deaf gene. Nature, 574, 465-466.
Gallego, J. (2016). Swedish Scientist Begins Editing Human DNA in Health Embryos. Futurism. Available from: https://futurism.com/swedish-scientist-begins-editing-human-dna-in-healthy-embryos.
International Commission on the clinical use of heritable human genome editing. (2020). Heritable Human Genome Editing: Consensus Study Report. Available from: https://www.nationalacademies.org/our-work/international-commission-on-the-clinical-use-of-human-germline-genome-editing.
Isasi, R., Kleiderman, E., & Knoppers, B. M. (2016). Editing policy to fit the genome? Science, 351(6271), 337-339.
Johnston, J. (2019). He Jiankui is going to jail. Would the U.S. criminally prosecute a rogue gene-editing researcher? Stat News. Available from: https://www.statnews.com/2019/12/31/he-jiankui-jail-prosecute-rogue-gene-editing-researcher/.
Knoppers, B. M., & Kleiderman, E. (2019). Heritable Genome Editing: Who Speaks for “Future” Children? The CRISPR Journal, 2(5), 285-292.
Kofler, N. (2019). Why were scientists silent over gene-edited babies? Nature, 566, 427.
Kolata, G., S.-L. Wee, and P. Belluck. (2018). Chinese Scientist Claims to Use CRISPR to Make First Genetically Edited Babies. The New York Times. Available from: https://www.nytimes.com/2018/11/26/health/gene-editing-babies-china.html.
Krimsky, S. (2019). Ten Ways in Which He Jiankui Violated Ethics. Nature Biotechnology, 37, 19-20.
Lander, E. S., Baylis, F., Zhang, F., Charpentier, E., Berg, P., Bourgain, C., et al. (2019) Adopt a moratorium on heritable genome editing. Nature, 567, 165-168.
Liang, P., Xu, Y., Zhang, X., Ding, C., Huang, R., Zhang, Z., et al. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6, 363–372.
Ma, H., Marti-Gutierrez, N., Park, S. W., Wu, J., Lee, Y., Suzuki, K., et al. (2017). Correction of a pathogenic gene mutation in human embryos. Nature, 548(7668), 413-419.
National Academies of Sciences, Engineering, and Medicine (NASEM). (2019). New International Commission Launched on Clinical Use of Heritable Human Genome Editing. Available from: https://www.nationalacademies.org/news/2019/05/new-international-commission-launched-on-clinical-use-of-heritable-human-genome-editing
National Academies of Sciences, Engineering, and Medicine (NASEM). (2017). Human Genome Editing: Science, Ethics, and Governance. Available from: https://www.nap.edu/catalog/24623/human-genome-editing-science-ethics-and-governance.
Nuffield Council on Bioethics. (2018). Genome editing and human reproduction: social and ethical issues. London: Nuffield Council on Bioethics. Available from: https://www.nuffieldbioethics.org/publications/genome-editing-and-human-reproduction.
Pera, M. F. (2017). Human embryo research and the 14-day rule. Development, 144(11), 1923-1925.
Reardon, S. (2019). World Health Organization panel weighs in on CRISPR-babies debate. Nature, 567, 444-445.
Servick, K. (2017). First U.S.-based group to edit human embryos brings practice closer to clinic. Science. doi: 10.1126/science.aan7197.
Townsend, B. A. (2020). Human genome editing: How to prevent rogue actors. BMC Medical Ethics, 21(1), 1-10.
Education vector created by freepik – www.freepik.com
