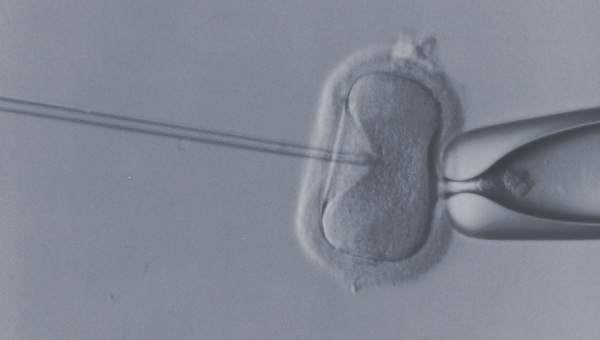
MRM Insights: Mitochondrial donation and the 2004 Assisted Human Reproduction Act

Forough Noohi
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Forough Noohi, Ph.D. Candidate in the Department of Human Genetics at McGill and member of the MRM Ethics group, is discussing mitochondrial donation and the 2004 Assisted Human Reproduction Act.
If you would like to learn more about mitochondrial replacement therapy in Canada, you can read Forough’s paper that was recently published in FACETS: Clinical translation of mitochondrial replacement therapy in Canada: a qualitative study of stakeholders’ attitudes.
Mitochondrial donation and the 2004 Assisted Human Reproduction Act
It has been twenty-eight years since the publication of Proceed with Care by the Royal Commission on New Reproductive Technologies.1 In a final report, the Commission drafted 293 recommendations which laid the foundation of the 2004 Assisted Human Reproduction Act (AHRA). The Act prohibits any practice that modifies the genome of “a human being or in vitro embryo such that the alteration is capable of being transmitted to descendants.”2 As such, mitochondrial donation or mitochondrial replacement therapy (MRT) is banned in both research and clinical contexts. While a periodic review of the AHRA was legally mandated in 2004, no such attempt has been made thus far.
MRT is a new type of in vitro fertilization that aims to prevent the transmission of mitochondrial diseases (matrilineal transmission) by replacing mutated mitochondrial DNA in unfertilized oocytes or zygotes with normal mitochondria from a healthy egg donor. Inherited mitochondrial diseases are severe and progressive disorders that have no cure.3 Currently, the gold standard for diagnosis and treatment of mitochondrial diseases are diagnostic genetic testing and supportive care. Predictive tests though, are unable to help many women at risk of transmitting severe mitochondrial diseases to their children.4
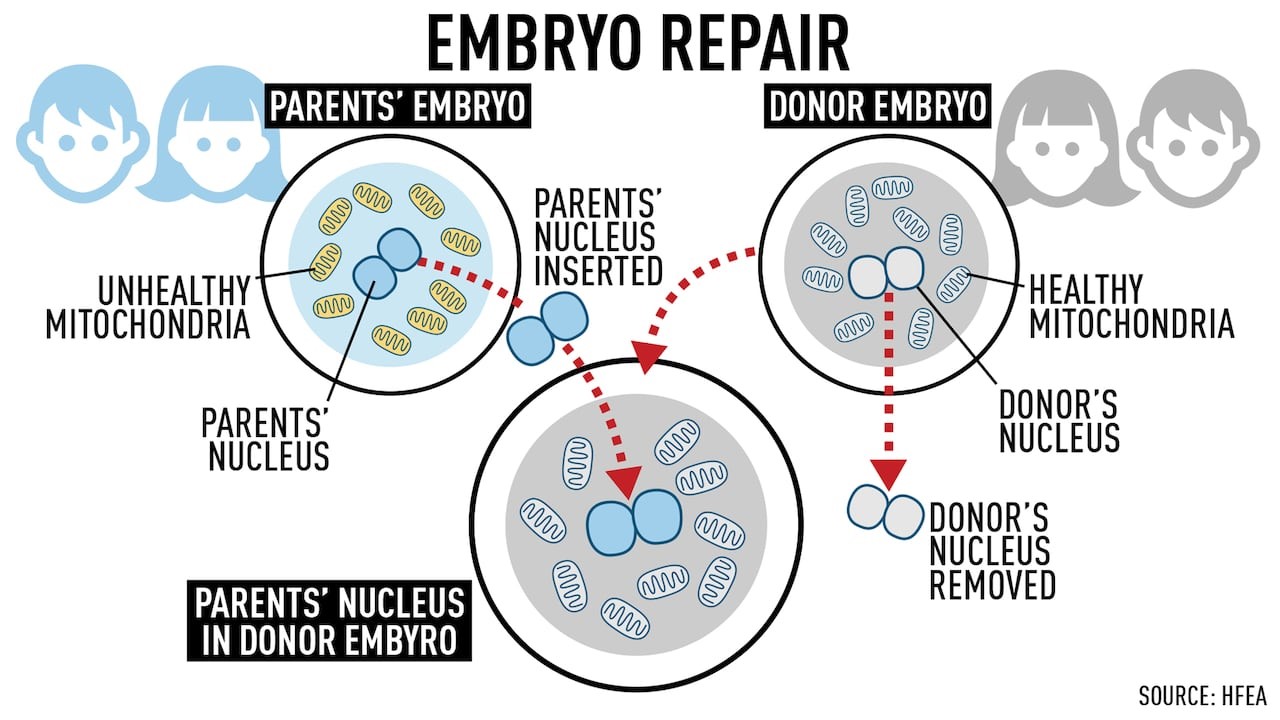
Jurisdictions with less strict or no reproductive laws are moving forward with MRT in the clinical context. Reportedly, seventeen babies have already been born using this technique in Mexico, Ukraine, and Greece.5,6,7
Now, more than ever, delving into the legitimacy of germline modifying techniques in the context of having healthy genetically related children may seem trivial in a society reordered by a global pandemic. It is, nonetheless, critical to acknowledge that a small number of Canadian families with heritable mitochondrial diseases are likely to seek out MRT.8 The review of the AHRA is long overdue. Investing in a broad public deliberation about a regulatory approach that is attentive to context is essential for preparing for a future in which technologies, such as MRT and CRISPR, advance to expand prospective parents’ reproductive options.
References:
1. Royal Commission on New Reproductive Technologies. 1993. http://publications.gc.ca/site/eng/9.699855/publication.html
2. Assisted Human Reproduction Act, S.C. 2004, c. 2. Section 5 (Prohibited Activities). Article (5)(1)(f). https://laws-lois.justice.gc.ca/eng/acts/a-13.4/page-1.html#h-6052
3. Ng YS and Turnbull DM. 2020. When to think about mitochondrial disease. Practical Neurology; 20:260-261. http://dx.doi.org/10.1136/practneurol-2020-002501
4. Wai T, Teoli D, Shoubridge EA. 2008. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40, 1484–1488. Nat Genet. 40(12):1484-8. https://doi.org/10.1038/ng.258
5. Zhang J, Liu H, Luo S, et al. 2017. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease, Reproductive BioMedicine Online;34 (4): 361- 368. https://pubmed.ncbi.nlm.nih.gov/28385334/
6. Darwin Life Nadiya. 2021. http://dl-nadiya.com/nuclear-transfers
7. Institute of life. 2021. https://www.iolife.eu/en/us/mst-research/
8. Noohi, Li, Joly. 2021. Clinical translation of mitochondrial replacement therapy in Canada: A qualitative study of stakeholders’ attitudes. FACETS. https://www.facetsjournal.com/doi/full/10.1139/facets-2020-0062
