MRM Insights – Biofabrication: a perspective on the future of personalized medicine

Dr. Megan Cooke

Dr. Rosenzweig
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Derek Rosenzweig, Assistant Professor in the Department of Surgery at McGill University, and Dr. Megan Cooke, Postdoctoral Fellow in his lab, are discussing biofabrication as a perspective on the future of personalized medicine.
Biofabrication: a perspective on the future of personalized medicine
Bringing a new therapeutic to market is an arduous task, which has been estimated to cost approximately 2 billion dollars, and may take over 10 years (DiMasi et al., J. Health Econo 2016, 47:p20-33). A study from 2013 revealed that about 75% of newly developed drugs failed to pass phase II clinical trials (Ringel et al., Nature Rev Drug Discov, 12:p901-902), while several news outlets report up to 90% failure rates. The poor clinical translation has mostly been attributed to lack of efficacy which is typically determined using in vitro cell lines cultured on 2D rigid plastic dishes or in small/large animal models which do not accurately recapitulate human physiology, pathology or immunity. New pre-clinical screening platforms that better mimic the complex tumor physiological microenvironment are necessary to enhance regulatory approval and clinical translation. This is particularly important for developing new treatments for painful degenerative diseases and various types of cancer patients. As a prime example, cancer is the leading cause of death in Canada accounting for 30% of all deaths (https://www.cancer.ca/en/). The Canadian Cancer Society has estimated more than 220,000 new cancer cases and over 82,000 deaths due to cancer for the year 2019.
Biofabrication can be described as the fabrication of complex, tissue-like constructs generated from a combination of living cells, biomaterials and other molecules, produced using automated technology such as bioprinting. Biofabrication is a fairly new development in the field of tissue engineering and is most often associated with 3D extrusion bioprinting – the layer-by-layer construction of three-dimensional structures using cell-seeded materials termed bioinks. A major advantage to using 3D bioprinting in biofabrication and tissue engineering is that there is a high level of control over spatial distribution of multiple cell types, materials and other factors in a reproducible manner. This approach can be used to incorporate micro-channels throughout the construct, which can be vascularized to enable fluid flow, nutrient uptake and waste removal. Many laboratories have demonstrated that such tissues provide clinically relevant, functional human tissues which can represent brain, breast, prostate, bone and lung cancers to name a few. While some researchers have their eyes set on using this technology in the future to reproduce/replace organs using a patient’s own stem cells, a much more practical and immediate application is already being used by academia and industry alike. High and medium throughput therapeutic compound library screening is readily being applied to 3D bioprinted human tissue models in 96- and 384-well plate formats. Such screening platforms often use immortalized human cell lines, but more recently evidence has emerged using patient-derived cells. Such constructs involve the spatial distribution of tumor cells, stromal cells and vascular endothelial cells. In many cases, these cells can be obtained from matching donors thereby presenting a physiological tumor microenvironment of that particular patient. This immediately opens the door toward personalized and precision medicine.
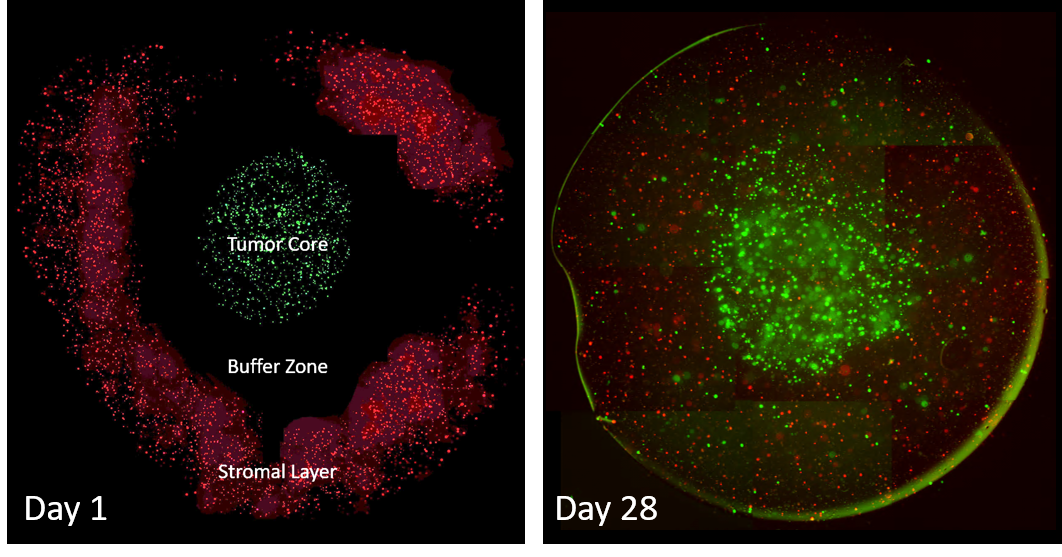
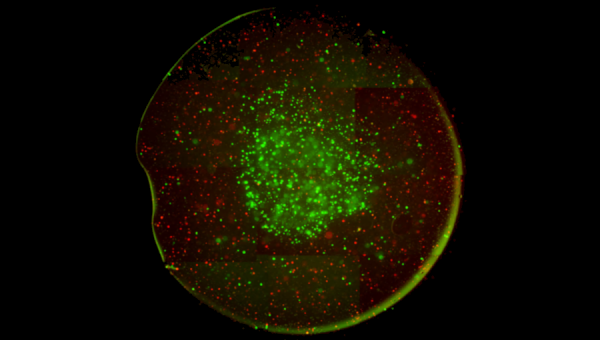
Tumor Micoenvironment Model. Using extrusion 3D bioprinting, we generate a “tumor core” with GFP-labeled triple-negative breast cancer cells surrounded by an empty gel “buffer zone”, which is also surrounded by a “stromal zone” containing Vybrant DiI labeled (red) primary human osteoblasts and nano-hydroxyapatite. At day 1, right after printing, the model has a bullseye shape. Over the course of 28 days, tumor cells grow into mutli-cellular spheroids and begin to migrate out toward the stromal layers. At the same time, stromal cells migrate in toward the tumor core. This metastasis-on-chip format is highly reproducible, can be made in 96-well plate format, and can include any number of cells/materials. We are now working on testing various therapeutics to block this cell growth and migration and will work toward using it with patient derived spine metastases cells and patient matching osteoblasts/stromal cells in an effort to identify personalized therapies.
In addition to being a valuable tool for therapeutic screening, biofabrication and 3D bioprinting also holds promise for studying mechanisms of tissue development and tissue pathology. For example, several laboratories around the world have used this approach for cartilage and bone repair and regeneration as well as cardiac muscle patches to name a few. At the heart of the approach lies the use of mesenchymal stem cells and induced pluripotent stem cells, often derived from patient donors. By appropriately designing the geometries of the constructs and cellular distribution, using the appropriate combination of stem cells and primary source cells and combing all with the most appropriate bioink, functional tissues can be generated to address various mechanistic questions. These constructs can also be looped back into therapeutic screening assays as well. With the increasing power and accessibility of biofabrication, the future of personalized, patient-centric medicine is looking brighter each day.
About the authors:
Dr. Derek Rosenzweig is a PI at McGill University, Department of Surgery and a scientist at the RI MUHC Injury, Repair and Recovery Program. His team has been developing a bone metastasis microenvironment model by printing patient-derived tumor cell core in soft hydrogel surrounded by normal human osteoblasts, stromal cells and endothelial cells in a stiffer hydrogel containing nano-hydroxyapatite. Microchannels are also incorporated for fluid flow. Novel therapeutic delivery devices are being screened against standard chemotherapeutics to block tumor cell proliferation and migration toward the bone-like environment. Dr. Cooke has played an integral role in development of this system.
Dr. Megan Cooke is a CIHR and FRQNT postdoctoral fellow. She has previously worked on the development of a novel bioprinting method to overcome the limitations of printing bioinks of very low viscosity, close to that of water. This relies on the use of a microgel-filled support bath, into which cell-seeded bioinks are printed prior to solidification. She is currently developing microgel baths to support the formation of vascular structures between gel particles. Using bioprinting methods, we can deposit materials, cells and cell spheroids with great control over their spatial distribution in 3D. This model will be used to investigate pro-angiogenic tumour responses as well as vascular abnormalities in bone fracture healing.
Illustrations:
Photo Credit: curtesy of Dr. Pouyan Ahangar and Dr. Megan Cooke
