MRM Insights: Targeting OXPHOS in Glioma Stem Cells

Laura Raco

Dr. Arezu Jahani-Asl
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Arezu Jahani-Asl, Senior Investigator, and Laura Raco, MSc. candidate in her lab, are discussing the role of OXPHOS in glioma stem cells.
Targeting OXPHOS in Glioma Stem Cells
Since their discovery, stem cells have changed the course of science and medicine by pushing the boundaries of what was once thought impossible. Their unique ability to generate various types of cells and tissues throughout the human body has quickly made them one of the most fascinating and complex cells to study and understand (Reya et al. 2001, Zakrerski et al. 2019). Regulation of stem cells arise from the expression of key transcriptional and epigenetic programs that allow these cells to remain in an undifferentiated state. Although the mechanisms by which these programs are fostered is still an ongoing area of research, it is clear that intrinsic and extrinsic signals from the microenvironment have a significant role to play in the formation and maintenance of these populations as well as in the timing of differentiation (Liebau et al. 2016). For instance, the stem cell niche is thought to be a significant mediator in stem cell fate such that niche cells provide the shelter and enable cell homeostasis to maintain a balance of self-renewal and differentiation. Furthermore, stem cell spatiotemporal regulation in relation to niche cells also plays a major role in the status and number of perpetuated cells (Moore & Lemishka 2006). Adding to their complexity of regulation, stem cells rely on different metabolic mechanisms to ensure their survival depending on the specific state that they are in. For instance, naive and differentiating Pluripotent Stem Cells (PSC) rely heavily on oxidative phosphorylation (OXPHOS) whereas primed pluripotent stem cells preferentially upregulate glycolysis (Shyh-Chang & Ng 2017). In light of this added complexity, it is however clear that mitochondrial metabolism is a significant driver of survival and stem cell fate. For instance, Reactive Oxygen Species (ROS) generation, Tricarboxylic Acid (TCA) metabolites production, pyruvate metabolism, NAD+/NAHD ratio and mitochondrial dynamics all have important roles in the fate regulation of stem cells (Chakrabarty & Chandel 2021).
Over the past decade the field of science has turned to stem cells and cells with stem-like properties for cancer research. Early experiments provided evidence of the possibility of the occurrence of stem-like cells in Acute Myeloid Leukemia (Lapidot et al. 1994). Quickly after evidence emerged that cells with stem-like properties, referred to as Cancer Stem Cells (CSC) were also identified in solid tumors (Singh et al. 2004, Prince et al. 2008, Al-Hajj et al. 2003). Now, CSCs are thought to be a key element in cancer growth, metastasis, recurrence and resistance to medical intervention. These cells are able to generate differentiated cells as well as maintain the stem cell pool through both asymmetric and symmetric division (Batlle et al. 2017). Although there is no one definitive mechanism as to how these specialized cancer cells arise, it is clear that mitochondrial function has a significant impact not only in cancer progression but in the occurrence and maintenance of CSCs (Vyas et al. 2016).
Previously established research in the domain of metabolism in cancer cells suggests that cancer cells rely heavily on glycolysis. There have been many proposals as to why this process might take place and pose a specific advantage to cancer cells but since this first observation, there has been evidence that not all cancer cells, including some with stem-like properties, function in this specific manner (Aston et al. 2018). This is similar to traditional PSC in that they present different metabolic preferences based on state or identity (Shyh-Chang & Ng 2017). Through research efforts into metabolism over the past years OXPHOS, in some cancer stem cells, has been shown to play a significant role in survival and fitness (Yadav et al., 2020). In 2020, Bonnay et al. demonstrated that oxidative phosphorylation is a defining characteristic of stem-like cells derived from Drosophila brain tumors. The upregulation of OXPHOS drives immortalization of Neural Stem Cells (NSC) and fosters an environment for tumorigenesis, while blocking OXPHOS impairs tumor formation (Bonnay et al. 2020). Further supporting the idea that OXPHOS may play a significant role in CSC of certain cancer types. Valle et al. 2020, described a method by which CSC derived from Pancreatic Ductal Adenocarcinoma (PDAC) can be maintained and enriched in culture through a forced switch to the use of OXPHOS. They were able to show that CSC may functionally require OXPHOS metabolism for chemoresistance, immune evasion, regulation of quiescence and self-renewal capabilities (Valle et al., 2020). The importance of OXPHOS in CSCs has also been shown in non-solid tumor cancers such as Acute Myeloid Leukemia (AML). Mubritinib, a drug previously identified as an ERRB2 inhibitor was found to localize to the mitochondrial in Leukemic Stem Cells (LSC) where it functionally inhibits OXPHOS and induces a switch to glycolysis in a distinct subset of AML cells which present poor patient prognosis. This drug functions by impacting the Electron Transport Chain (ETC), specifically by interacting with Complex I to impair mitochondrial respiration. Upon analysis of Mubritinib sensitive cells, the scientists were able to assess that LSC, specifically those from poor patient prognosis background are most sensitive and correlate highly with increased expression of genes relating to mitochondrial respiration (Baccelli et al., 2019). Specifically in the realm of brain tumors, CSC derived from gliomas have been found to rely heavily on OXPHOS even in the presence of substrate for glycolysis suggesting that OXPHOS may be endowing these cells with specific survival advantages (Vlashi et al., 2011). With a growing body of evidence suggesting that OXPHOS plays a role in tumorigenesis of various types of solid and hematopoietic cancers, our lab has sought to investigate the mechanism of this occurrence specifically in glioblastoma.
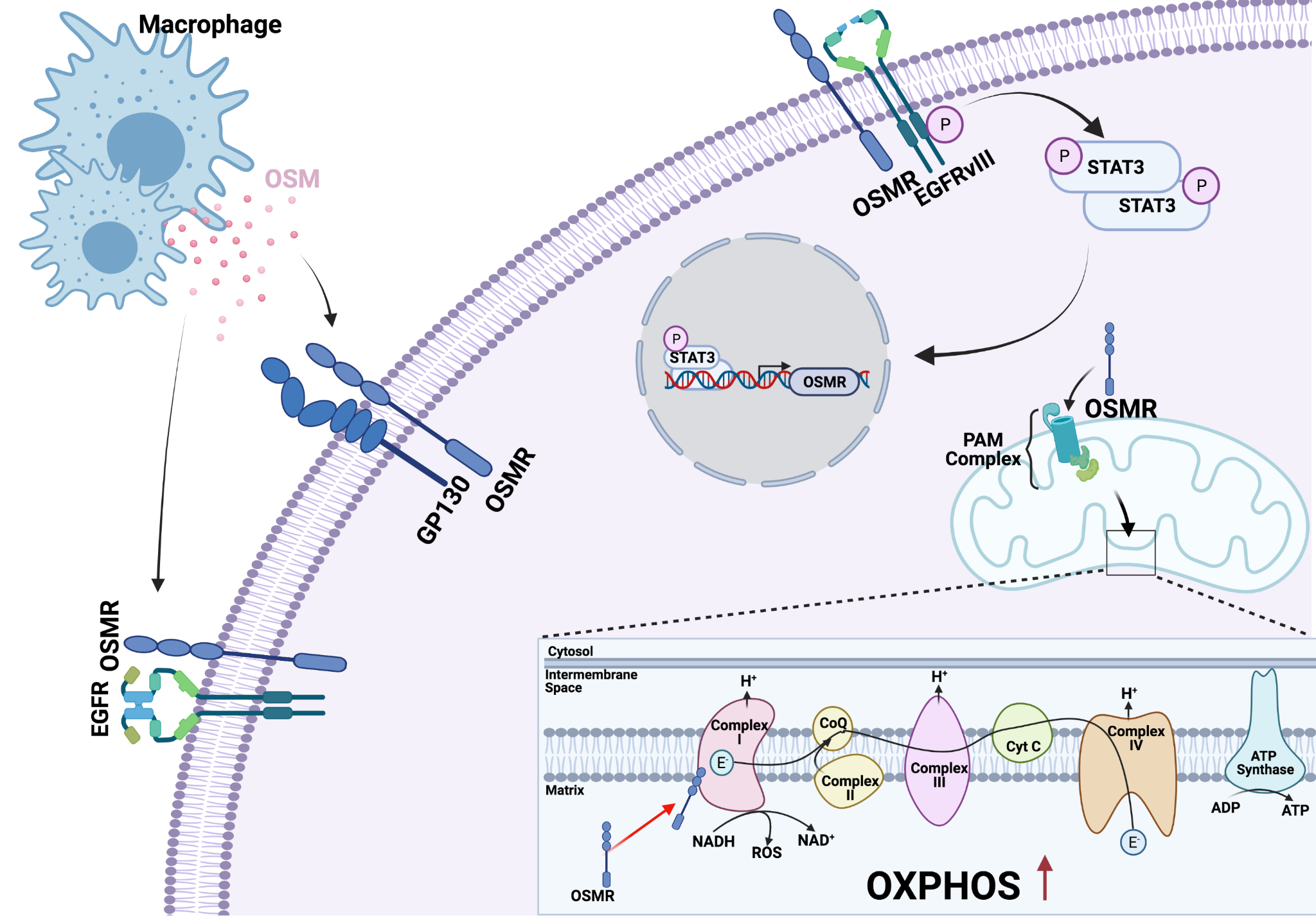
OSMR, a novel key player in the regulation of OXPHOS and glioblastoma resistance to therapy
Glioblastoma is one of the most aggressive and lethal types of cancer. Over the past decade there have been countless efforts to find new treatments, however the previously established treatment options consisting of surgical resection, radiotherapy and chemotherapy are still considered the standard of care across the board. Still, these tumors present high levels of recurrence which has come to be the focus of current research efforts (Shergalis et al., 2018). The high level of recurrence is oftentimes attributed to a small subset of Glioma Stem Cells (GSC) which have the ability to propagate new tumor formations even after intense medical intervention. Adding to their complexity, these types of cells have been found to be heterogeneous in nature (Atashzar et al., 2020). Recently, our lab has identified a key player in the regulation of OXPHOS in CSC derived from glioblastoma tumors (Sharanek et al., 2020). Oncostatin M Receptor (OSMR) is a cytokine receptor that has gained more attention in recent years. In 2016 our laboratory made the novel discovery that OSMR functions as an essential player in glioblastoma progression through direct interaction with the oncogenic EGFRvIII in human brain tumor stem cells (BTSC) and murine astrocytes. This interaction induces the phosphorylation of STAT3 in BTSC which in turn promotes the expression of OSMR, thereby effectively generating a positive feedback loop contributing to tumorigenesis. Knockdown of OSMR in vivo and in vitro attenuated CSC proliferation and tumor growth and extended lifespan of xenograft mice. Importantly, analysis of the patient database revealed that elevated expression of OSMR is significantly correlated with poor patient prognosis (Jahani-Asl et al., 2016).
In follow up studies, we recently discovered that OSMR is targeted to the mitochondria where, after translocation into the mitochondrial matrix, interacts with Complex I of the electron transport chain (ETC) to promote respiration of BTSCs. This discovery came by looking at the mechanisms by which OSMR contributes to glioblastoma tumorigenesis outside of its role as an EGFRvIII co-receptor. Its specific interaction with NDUFS1 and NDUFS2 of Complex I allowed for the hypothesis that OSMR was functionally impacting the ETC. Further experimentation did in fact show that OSMR was impacting mitochondrial respiration such that in the presence of OSMR CSCs have a significantly higher level of oxygen consumptions pointing to the notion that OSMR may regulate OXPHOS in this population. Knockdown of OSMR, decreased OXPHOS and induced a turn to glycolysis. Finally, our lab was able to demonstrate that brain CSC reliance on OXPHOS is also impacting cellular sensitivity to Ionizing Radiation (IR), such that these cells present higher ROS and higher sensitivity to IR in OSMR knockdown cell lines (Sharanek et al., 2020). This data points to the importance of OXPHOS in GSC specifically and suggests a potential method in which heterogeneous GSC/CSC populations may be identified based on drivers of key metabolic functions.
Although specifically targeting OXPHOS directly through inhibitors in cancer has raised some questions regarding toxicity to neighbouring cells as well as key immune cells (Weiss 2020), identification and targeting of key players of OXPHOS opens new avenues for potential therapeutic interventions converging on the mitochondria. Our recent discoveries propose a model whereby targeting of OSMR in combination with present standards of care, can have great therapeutic potential to alleviate CSC and tumour resistance to IR. Importantly, most recently, the cytokine OSM, produced by macrophages, was shown to interact with its receptor OSMR on cancer cells to activate STAT3 and induce the transition of glioblastoma cells toward mesenchymal-like states (Hara et al. 2021). Thus, OSMR targeted therapies may hold promise for controlling the immune microenvironment, modulating CSC metabolism, and suppressing feedforward mechanisms on CSC and tumour types that rely on EGFRvIII/STAT3 signalling for invasiveness.
References
Reya, T. et al. Stem cells, cancer and cancer stem cells. Nature 414 105-111 (2001)
Zakrzewski, W. et al. Stem cells: past, present and future. Stem Cell Res. Ther. 10, 68 (2019).
Liebau, S. et al. Factors regulating stem cell biology in development and disease. Stem Cells Int. 7170642 (2016)
Moore, K.A. & Lemischka, I.R. Stem cells and their niches. Science 311, 1880-1885 (2006)
Shyh-Chang, N. & Ng, H.H. The metabolic programming of stem cells. Genes Dev. 31, 336-346 (2017).
Chakrabarty, R.P & Chandel, N.S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 28, 394-408 (2021)
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645-468 (1994).
Singh, S.K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Prince, M.E.P. et al. Cancer stem cells in head and neck squamous cell cancer. J. Clin. Oncol. 26, 2871-2875 (2008).
Al-Hajj, M. et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 3983–3988 (2003).
Batlle , E. et al. Cancer stem cells revisited. Nat Med. 23 1124-1134 (2017).
Vyas, et al. Mitochondria and cancer. Cell 166, 555-566 (2016).
Liberti, M.V. & Locasale J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 41, 211-218 (2016).
Ashton, T.M et al. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 24, 2482-2490 (2018)
Yadav, U.P. et al. Metabolic adaptations in cancer stem cells. Front. Oncol. 10, 1010 (2020).
Bonnay, F. Oxidative metabolism drives immortalization of neural stem cells during tumorigenesis. Cell 182, 1490-1507 (2020)
Valle, S. et al. Exploiting oxidative phosphorylation to promote stem and immunosuppressive properties in pancreatic stem cells. Nat. Commun. 11, 5262 (2020).
Baccelli, I. et al. Mubritinib targets the electron transport chain complex I and reveals the landscape of OXPHOS dependency in acute myeloid leukemia. Cancer Cell 36, 84-99 (2019).
Vlashi, E. et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl. Acad. Sci. USA 108, 16062-16067 (2011).
Shergalis, A. et al. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 70, 412-445 (2018).
Atashzar, M.R. et al. Cancer stem cells: A review from origin to therapeutic implications. J. Cell. Physiol. 235, 790-803 (2020).
Sharanek, A. et al. OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation. Nat. Commun. 11, 4116 (2020).
Jahani-Asl, A. et al. Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nat Neurosci. 19, 798-806 (2016).
Weiss, J.M. The promise and peril of targeting cell metabolism for cancer therapy. Cancer Immunol. Immunother. 69, 255-261 (2020).
Hara, et al. Interaction between cancer cells and immune cells drive transition to mesenchymal-like states in glioblastoma. Cancer Cell 39, 779-792 (2021)
