MRM Insights: Asymmetric cell division and cancer cell stemness

Henry Yu

Dr. Moulay Alaoui-Jamali
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Moulay Alaoui-Jamali, Professor and Senior Investigator in the Department of Medicine and Oncology at McGill University and his lab member Henry Yu (Ph.D. candidate) are discussing the role of asymmetric cell division and centrosome amplification in the regulation of cancer cell stemness and heterogeneity.
Centrosome asymmetry as a stemness-derived driver of cancer chromosomal instability and intratumoral heterogeneity
Intratumoral heterogeneity is common in most cancer types with important prognostic and therapeutic implications. The presence of genetically distinct cancer cell subpopulations within a tumor mass is considered among major drivers of cancer progression, as well as resistance to therapeutics/relapses. Advanced genome-wide and single-cell DNA sequencing analyses of patient-derived cancer tissues and isolated tumor cells yielded comprehensive portraits of the diversity of genetic alterations among distinct cancer cell variants within a tumor mass1.
In a broader context, genetic diversity is contributed by genomic instability due to oncogene-induced DNA damage and dysfunction of DNA repair2. Such diversity can be further exacerbated by selective pressures exerted by metabolic and chemotherapy stress3. Genomic instability leads to chromosome instability (CIN) and aneuploidy (defined as gains, losses or rearrangements of chromosomal regions), enabling the macro-evolutionary leaps required for tumor evolution and metastasis4. Chromosomal aberrations also commonly arise during culture of human-induced pluripotent stem cells (hiPSC) and human embryonic stem cells (hESC), or somatic stem cells and represent a major obstacle in their use for regenerative medicine5,6. Interestingly, stem cells derived from different tissues have particular chromosome arms that are predisposed to chromosomal aberrations6, perhaps due to distinct chromatin architectures associated with certain states of differentiation7.
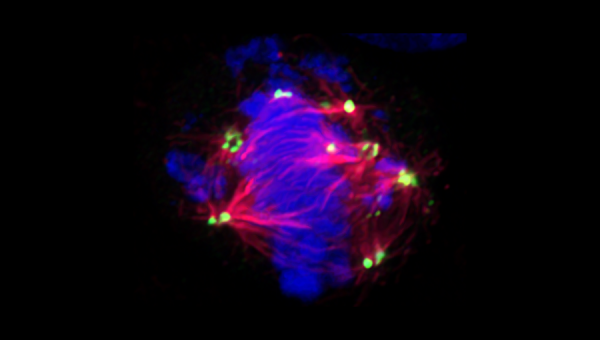
CIN in stem cells and cancer can both arise due to centrosome amplification. Centrosome amplification, the acquisition of >2 centrosomes by a cell, is a frequent feature of cancer and is generally more common in high-grade cancers8. Centrosomes are the animal cell’s microtubule-organizing centers (MTOC). They are a pair of organelles that, through the formation of a gamma-tubulin ring complex, nucleate microtubules, thus dictating cell shape, polarity, motility, spindle formation, chromosome segregation, and ultimately cell division9. Mechanistically, supernumerary centrosomes cause aneuploidy by increasing the number of spindle poles formed during mitosis; this occurs due to increased merotelic attachments – a type of error in spindle-chromosome binding whereby a single kinetochore is mistakenly attached to microtubules radiating from multiple spindle poles, resulting in lagging chromosomes during anaphase10.
Centrosomes are structurally amorphous, composed of a pair of centrioles embedded within “pericentriolar materials” (PCM) that contain ~100 different proteins11. A non-proliferating cell contains one centrosome made of two centrioles, which is typically anchored at the basement of the primary cilia9. Upon entering the cell cycle, the two centrioles disengage and mature by replicating a second centriole while accumulating PCM11. In most cells, the maturation of the two centrosomes occurs at similar rates. In stem cells models, however, only one centrosome accumulates PCM during interphase, resulting in a slight temporal asymmetry in the formation of the two mitotic spindles12. These unique features are of great importance to a specialized mode of cell division known as asymmetrical cell division (ACD).
Centrosome asymmetry coordinates stem cell asymmetrical cell division
Under physiological conditions, tissue morphogenesis is dictated by a balance between stem cell self-renewal and differentiation and is tightly regulated through symmetric and asymmetric cell divisions (figure 1). ACD, which is driven by both extrinsic (originating from outside the cell such as paracrine factors secreted by the stem cell niche) or intrinsic factors (present within the stem cell itself), is a critical determinant for mammalian stem cell fate by regulating the production of stem cells and differentiating daughter cells (reviewed in13).
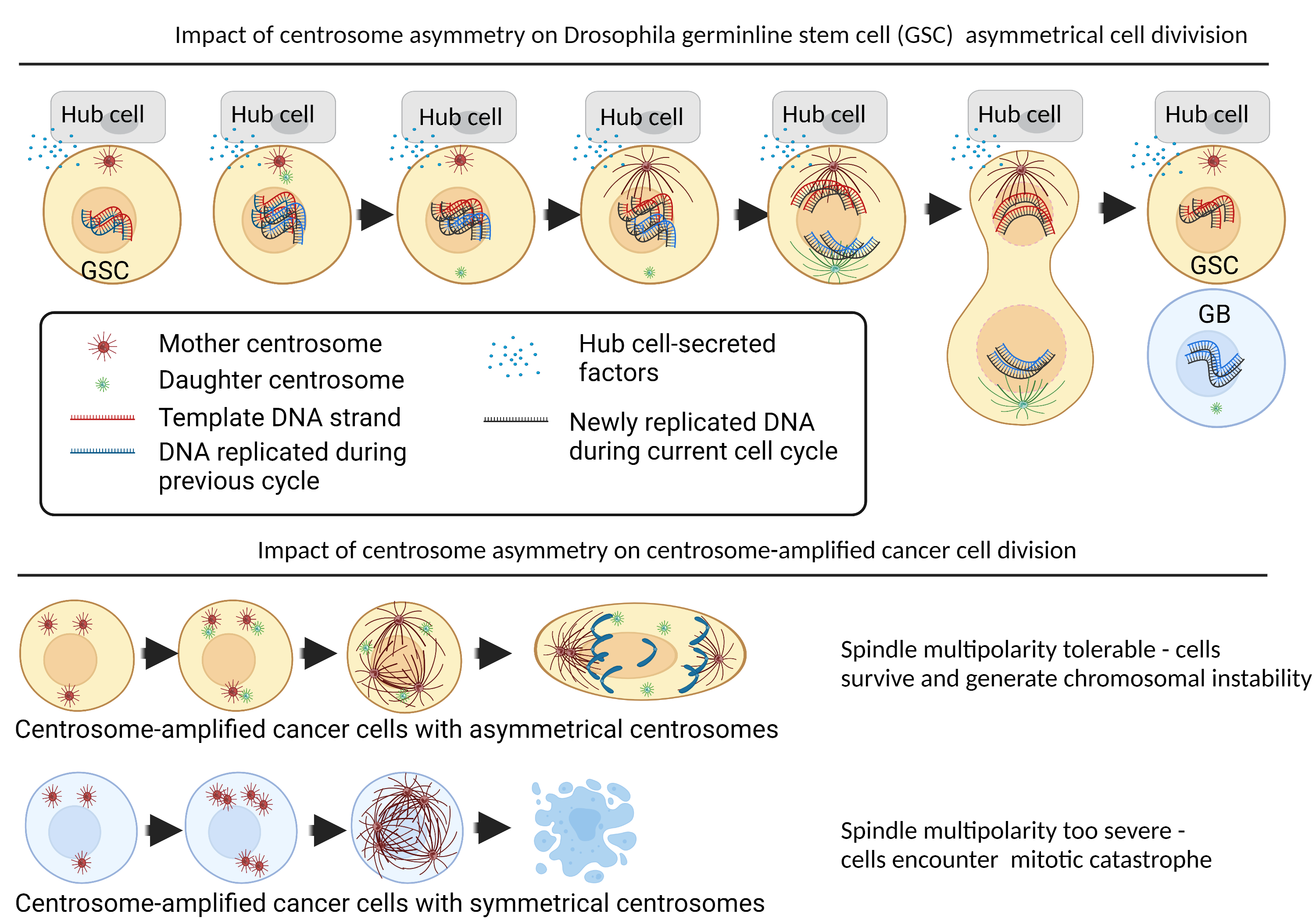
Figure 1: Impact of centrosome asymmetry in stem cells and centrosome-amplified cancer cells. Asymmetrical cell division is driven by extrinsic factors originating from outside the cell (such as by paracrine factors secreted by the stem cell niche) and intrinsic factors originating from inherent asymmetries present within the stem cell itself. Much of the study of asymmetrical cell division is conducted in Drosophila models. In Drosophila germline stem cells (GSC), the GSCs interact with the stem cell niche (or “hub cells”) via adherens junctions. The hub cell secretes factors such as Dpp and Upd to dictate the organization of astral microtubules (microtubules that extends from the centrosomes to the cortex of the cell membrane) to dictate the positioning of the centrosome and the orientation of the mitotic spindle. This mechanism has two known functions. Firstly, it maintains the position of the GSC close to the niche, while the second daughter cell – the gonial blast (GB) is isolated from the niche-cues and thus driven to differentiation14. Secondly, the asymmetrical centrosomes coordinate processes at the centromere which allows the GSC to retain all the template DNA during asymmetrical cell division, which would reduce the propagation of DNA-copy errors12. In our unpublished findings, we found that certain cancer cells could leverage mechanisms highly reminiscent of Drosophila stem cell centrosome patterns. Unlike stem cells, these cancer cells exhibit a high frequency of centrosome amplification. Cancer cells with asymmetrical centrosomes could maintain spindle multipolarity at a tolerable level to be able to complete mitosis, resulting in cells surviving with a high degree of chromosomal instability. Cancer cells which symmetrical centrosomes were more prone to mitotic catastrophe, and thus were eliminated before they could propagate chromosomal instability. Figure created using Biorender.com.
The decision on which of the two daughter cells will acquire stemness traits during ACD depends in part on cell polarizing cues in the niche13. For instance, in Drosophila neuroblasts, cell-fate determinants are intrinsically segregated to two cell poles during interphase based on polarity cues from the epithelial cell layer. These polarized fate-determinants (e.g., Par3/par6) capture microtubules emanating from one specific centrosome (e.g., daughter centrosome) to direct the mitotic spindle parallel to the apical-basal axis.
Presently, centrosome asymmetry supports the maintenance of the stem cell phenotype in two key manners. First, by controlling spindle orientation, centrosome asymmetry can ensure that only one of the daughter cells remains near the stem cell niche (e.g., Hub cells), while the second daughter cell is positioned further away, and therefore predisposed to differentiation through the loss of the environmental cue necessary for sustaining stem cell identity14. Secondly, centrosome asymmetry coordinates with proteins located at the centromeres (specialized region of the chromosome which attaches to the mitotic spindle) to ensure that the daughter cell with stem cell identity also inherits all the template (original) DNA strand, thus limiting the propagation of DNA replication errors12.
Chromosomal instability arises when mitosis succeeds despite centrosome amplification
Centrosome amplification can arise from several non-mutually exclusive mechanisms. Errors in the centrosome cycle can lead to centriole overduplication or de novo centriole genesis, while errors in cytokinesis can generate polyploid cells with extra pairs of centrosomes15. Although the formation of multipolar spindles promotes CIN/aneuploidy, multipolar mitosis (where a cell divides into three or more daughter cells) almost always leads to unviable cells16. Cancer cells avert this catastrophe by preventing multipolar spindles from progressing beyond metaphase; instead, a process called “centrosome clustering” is utilized by cancer cells to assemble mitotic spindles into one of two poles17. The resultant “pseudobipolar spindle” retains merotelic attachments between spindles/kinetochores without committing to multipolar mitosis, thus effectively propagating CIN/aneuploidy while generating viable daughter cells.
Centrosome asymmetry meets centrosome amplification in cancer stem cells
Although the precise nature of cancer stem cells has been difficult to pinpoint, particularly in vivo, there is ample evidence supporting the notion that certain cancer cells manifest traits that are physiologically restricted to stem/progenitor cells18. In the context of centrosomes, this raises an interesting question as to what happens when both centrosome asymmetry and centrosome amplification co-manifest in malignant cells.

Figure 2: Inhibition of centrosome asymmetry promotes maturation of supernumerary centrosome. Top: Cancer cells can simultaneously display centrosome asymmetry and amplification, whereupon some, but not all, centrioles fail to accumulate pericentriolar material during interphase. Bottom: Inhibition of centrosome asymmetry factors, characterized as factors that prevent centrosome asymmetry and asymmetrical cell division in cells with two centrosomes, produces supernumerary centrosomes that mature evenly.
In unpublished work, using anaplastic thyroid cancer, a highly dedifferentiated and aneuploid malignancy, we show that centrosome asymmetry can co-exist alongside centrosome amplification and is a property that cancer cells can leverage to promote CIN. Cancer cells achieve this aneuploidy by using centrosome-asymmetry promoting mechanisms to limit the maturation of supernumerary centrosomes during early mitosis (figure 2: top panels, demonstrating the abundance of centrioles that fail to replicate and acquire PCM), which is sufficient to delay the activation of supernumerary centrosomes (figure 3: top panels, demonstrating the presence of centrosomes with weak spindle forming capacity). Targeting centrosome asymmetry-factors limited both CIN and stem-like features (figures 2-3, bottom panels). Our findings illustrated a causal link between centrosome asymmetry and CIN in the context of cancer.

Figure 3: Inhibition of centrosome asymmetry promotes multipolar over pseudobipolar spindles. Top: Cancer cells displaying both centrosome asymmetry and centrosome amplification can effectively form pseudobipolar spindles by limiting the activation of supernumerary centrosomes. Bottom: Inhibition of centrosome asymmetry factors re-activates centrosome resulting in increased spindle multipolarity.
In summary, the understanding of how stem cells balance self-renewal and production of differentiating cells is a key element in our understanding of stem cell features in cancer and their contribution to intratumoral heterogeneity. Though spindle orientation and differential segregation of centrosomes/centromere/DNA in asymmetrically dividing cells is an important mechanism for specifying cell fate decisions, there remain multiple unsolved gaps and challenges that need to be addressed in the cancer field before implementing clinical translation strategies. Undoubtedly, studies to address relationships between cancer cell plasticity, centrosome aberrations (known to be highly tissue/cell context-dependent), and inherent versus acquired genetic cell heterogeneity are of tremendous importance.
References
1. Chung, W., et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nature communications 8, 1-12 (2017).
2. Burrell, R.A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338-345 (2013).
3. Kinker, G.S., et al. Pan-cancer single-cell RNA-seq identifies recurring programs of cellular heterogeneity. Nature genetics 52, 1208-1218 (2020).
4. McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613-628 (2017).
5. Mayshar, Y., et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell stem cell 7, 521-531 (2010).
6. Ben-David, U., Mayshar, Y. & Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell stem cell 9, 97-102 (2011).
7. Dixon, J.R., et al. Chromatin architecture reorganization during stem cell differentiation. Nature 518, 331-336 (2015).
8. Chan, J.Y. A clinical overview of centrosome amplification in human cancers. International journal of biological sciences 7, 1122 (2011).
9. Nigg, E.A. & Stearns, T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nature cell biology 13, 1154-1160 (2011).
10. Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1786, 32-40 (2008).
11. Jakobsen, L., et al. Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. The EMBO journal 30, 1520-1535 (2011).
12. Ranjan, R., Snedeker, J. & Chen, X. Asymmetric centromeres differentially coordinate with mitotic machinery to ensure biased sister chromatid segregation in germline stem cells. Cell stem cell 25, 666-681. e665 (2019).
13. Yamashita, Y.M., Mahowald, A.P., Perlin, J.R. & Fuller, M.T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science (New York, N.Y.) 315, 518-521 (2007).
14. Venkei, Z.G. & Yamashita, Y.M. Emerging mechanisms of asymmetric stem cell division. Journal of Cell Biology 217, 3785-3795 (2018).
15. Fukasawa, K. Centrosome amplification, chromosome instability and cancer development. Cancer letters 230, 6-19 (2005).
16. Ganem, N.J., Godinho, S.A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278-282 (2009).
17. Quintyne, N.J., Reing, J.E., Hoffelder, D.R., Gollin, S.M. & Saunders, W.S. Spindle multipolarity is prevented by centrosomal clustering. Science (New York, N.Y.) 307, 127-129 (2005).
18. Kreso, A. & Dick, J.E. Evolution of the cancer stem cell model. Cell stem cell 14, 275-291 (2014).
