MRM Insights: Autophagy in stem cell health and disease

Cordell VanGenderen

Dr. Natasha Chang
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Dr. Natasha Chang, Assistant Professor in the Department of Biochemistry at McGill University and her lab member Cordell VanGenderen (Ph.D. candidate) are discussing autophagy in stem cell health and disease.
Autophagy in stem cell health and disease
Autophagy (literally meaning self-eating) is the process through which cells break down their internal components to recycle the constituent biomolecules. Every cell has a basal level of autophagy, which can be elevated in response to stimuli including nutrient deprivation and the buildup of reactive oxygen species (ROS). Previous research in terminally differentiated cells has revealed a critical role for autophagy in the maintenance of cellular health through the removal of damaged organelles and proteins, as well as providing nutrients and energy in times of metabolic stress. Autophagy itself refers to three distinct mechanisms: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy is the most studied and best understood of these three, and for the purposes of this article will be referred to as autophagy. Briefly, this specific form of autophagy involves the formation of a double-membrane structure (known as a phagophore) that envelops the cargo to be degraded (Figure 1). Completion of the phagophore generates a vesicle known as the autophagosome, which then merges with a lysosome, forming the autolysosome. Enzymes from the lysosome then break down the contents of the autolysosome and the constituent components are recycled. This form of autophagy can be cargo selective, as seen in the case of mitophagy, where mitochondria are specifically targeted by the autophagosome for degradation (Parzych & Klionsky, 2014). Defects in autophagy have been observed in a variety of diseases, including neurodegenerative disorders, heart disease, and myopathies (Wirawan et al., 2011).
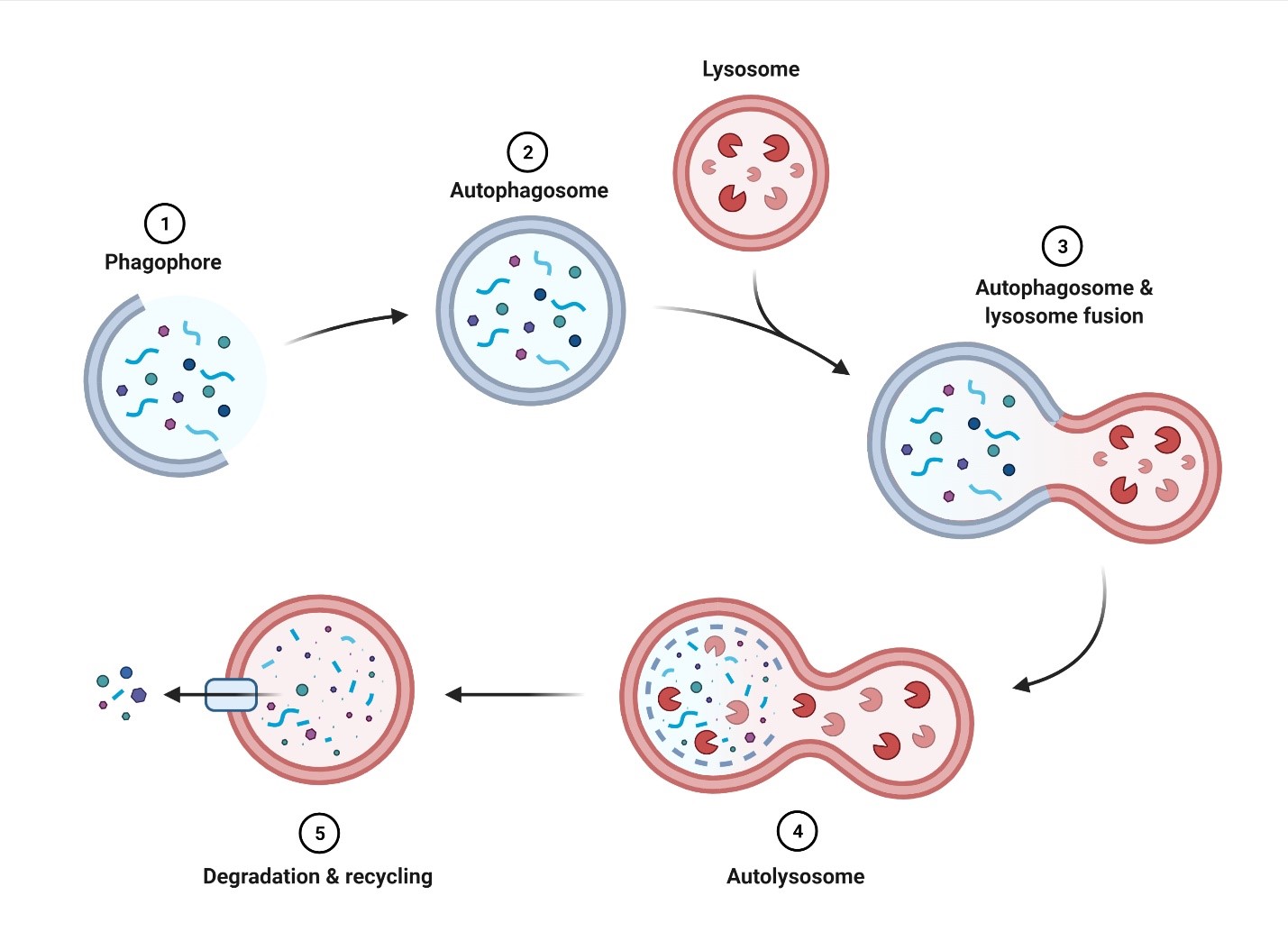
Figure 1. Overview of autophagy. 1) Elongation of the phagophore surrounding the cargo to be degraded. 2) Complete enclosure of the cargo results in the formation of the autophagosome. 3) Fusion of the autophagosome and lysosome. 4) Formation of the autolysosome upon complete fusion of the autophagosome and lysosome. 5) Cargo within the autolysosome is degraded to be reused by the cell. Figure was made with BioRender.com.
In recent years, there has been a growing appreciation for the role that autophagy plays in the biology and function of multipotent stem cells, and the potential implications of this relationship for disease beyond the general maintenance of cell health. Multipotent stem cells are defined as those which retain the self-renewal capacity of pluripotent stem cells but are restricted in their capacity to differentiate, being confined to one (or a few) lineages (Mirzaei et al., 2018). In 2016, García-Prat et al. discovered that autophagy is a vital process for the maintenance of stemness in muscle stem cells, which are also known as satellite cells (García-Prat et al., 2016). During homeostasis, satellite cells (as is typical with multipotent stem cells) remain in quiescence, a reversible state of cell cycle arrest. Upon stimulation (for instance muscle injury), they become activated and commit to myogenic differentiation. It was discovered that by directly inhibiting autophagy through satellite cell-specific deletion of the key autophagy gene Atg7, satellite cells came out of their quiescent state and entered senescence. Senescence is an irreversible state of cell cycle arrest, thus rendering satellite cells unable to activate, proliferate, and participate in muscle repair. The loss of autophagy induces an elevation in oxidative stress resulting from the buildup of ROS, a consequence of the accumulation of damaged mitochondria. In satellite cells, this results in the expression of p16INK4, which inhibits cyclin-dependent kinase 4 to induce cell cycle arrest and subsequent senescence. In aged satellite cells, they were able to reverse the senescent state through treatment with rapamycin, an inhibitor of the mammalian target of rapamycin complex I (mTORC1) and inducer of autophagy. Autophagy also serves to support other key functions in multipotent stem cells that are important for their ability to differentiate. Firstly, autophagy is required to meet the energetic demands necessary for stem cells as they undergo activation (Tang & Rando, 2014). Secondly, autophagy is crucial for the cellular remodelling that occurs as stem cells undergo differentiation, which has been observed in cardiac progenitor cells, hematopoietic stem cells, and germ cells (Huang et al., 2020; Joshi & Kundu, 2013; Lampert et al., 2019).
The connection between autophagy and stem cells is evident in age-related diseases. Continuing the discussion on muscle stem cell dysfunction, sarcopenia is the age-related loss of skeletal muscle and function. When examining satellite cell function in old (20-24 months old) vs geriatric mice (28-32 months), Sousa-Victor et al. performed a muscle injury and regeneration experiment to study subsequent satellite cell activation and muscle repair. The impaired regenerative capacity of the geriatric mice was not due to the number of satellite cells, as old and geriatric mice exhibited similar numbers of satellite cells. However, geriatric mice exhibited a greater prevalence of satellite cells in senescence, demonstrating that the inability to activate satellite cells leads to inefficient muscle regeneration that corresponds with sarcopenia (Sousa-Victor et al., 2014). Coupled with the results from García-Prat et al., which revealed that autophagy is important for preventing senescence in satellite cells, it is clear that the age-related decline in satellite cell autophagy leads to impaired muscle regeneration and results in muscle loss.
Another age-related disease directly impacted by stem cell autophagy is osteoporosis. In 2020, Yuan et al. demonstrated a connection between the loss of autophagy in hematopoietic stem cells (HSCs) with osteoporosis (Yuan et al., 2020). They observed a decrease in the red blood cell count of osteoporosis patients, indicating a disruption to HSC differentiation and proliferation. Upon examination of bone marrow samples from these patients, they found a significant reduction in the expression of autophagic genes. A follow-up experiment involving genetic deletion of Atg7 in mouse HSCs (but not the terminally differentiated lineages) resulted in severe bone loss and disrupted bone homeostasis, thus indicating a link between HSC autophagy with osteoporosis.
In Duchenne muscular dystrophy (DMD), a muscle degenerative disease that we study in the Chang laboratory, autophagy was found to be impaired in mature muscle cells of mdx mice, a murine model of DMD (Palma et al., 2012). However, at the time the potential role of satellite cells in DMD disease progression was not well understood. In 2015, Dumont et al. discovered that mdx satellite cells were intrinsically impaired and that satellite cell dysfunction is a contributing mechanism in DMD (Dumont et al., 2015). Dystrophic satellite cells exhibit impaired asymmetric stem cell division and favor self-renewal over commitment leading to reduced numbers of progenitor cells. While the contribution of stem cell autophagy in the context of DMD has not been clearly established, we hypothesize that impaired autophagy is playing a role in satellite cell dysfunction in DMD, which represents a key research focus in our lab.
The overactivity of autophagic pathways in multipotent stem cells has also been connected to disease. Within the liver reside hepatic stellate cells, which remain quiescent until their activation in response to liver injury, a process that relies on autophagy (Thoen et al., 2011). Upon activation, stellate cells begin to differentiate into myofibroblasts, which are the primary source of collagen in the extracellular matrix of the liver (ECM). The accumulation of ECM collagen results in liver fibrosis contributing to multiple chronic liver diseases (Higashi et al., 2017). During liver fibrosis, several signalling pathways are dysregulated in a manner that promotes autophagy including transforming growth factor β, HMGB1 (high-mobility group box-1), and IGFBP-rP1 (insulin-like growth factor-binding protein-related protein-1). Therefore, overactivation of pro-autophagy signaling within stellate cells results in their inappropriate activation and differentiation, contributing to chronic liver disease (Li et al., 2018; Zhang et al., 2021; Zhou et al., 2020).
Research over the last several years has established a clear trend, that disruption of autophagy in adult stem cells contributes to a myriad of diseases. This mechanistic insight has opened the door for therapeutic opportunities. There are many pharmacological agents which are capable of modulating autophagy, whether directly or indirectly. Rapamycin, which is the most well-known (as well as the plethora of rapamycin mimetics referred to as rapalogues) acts through inhibition of mTOR (Blagosklonny, 2019). mTOR is a central regulator of cell growth, serving as the kinase subunit of mTORC1 and mTORC2 complexes. In response to nutrient abundance, these protein complexes become active and phosphorylate a multitude of proteins to induce cell growth, including critical autophagy players ULK1 and transcription factor EB (TFEB). Phosphorylation of ULK1 and TFEB by mTORC1 inhibits their activity and prevents autophagy. ULK1 is responsible for forming the ULK1 initiation complex which induces phagophore formation, while TFEB induces lysosome biogenesis (Jung et al., 2010; Raben & Puertollano, 2016). An alternative method of inducing autophagy is through the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), which phosphorylates and negatively regulates mTOR while positively regulating ULK1 and TFEB (Jung et al., 2010). AMPK can be activated by metformin, a drug for type 2 diabetes that inhibits complex I of the mitochondrial respiratory chain to increase the amount of AMP, thereby stimulating AMPK activity (Kim et al., 2016). It is important to note that mTOR and AMPK are both broad metabolic regulatory proteins that impact a wide array of proteins to induce stress responses and autophagy (AMPK) or inhibit them (mTOR). Therefore, the targeting of these metabolic kinases may lead to undesirable consequences. For example, rapamycin is a well-documented immune modulator and is used to prevent organ rejection (Blagosklonny, 2019). An alternative approach is to directly target the autophagy machinery itself, of which there are nearly 100 proteins specifically involved in autophagy. One potential target is the ULK1 initiation complex. The pharmacological agent LYN-1604 has been identified as an ULK complex agonist, and when tested in a triple negative breast cancer trial was found to induce autophagy-related cell death (Xiang et al., 2020). While it is unlikely that a single agent will be able to treat all of the diseases discussed here, nonetheless fundamental studies that aim to uncover the role of stem cell autophagy in disease will provide important mechanistic insight and identify novel targets that modulate autophagy for regenerative medicine approaches.
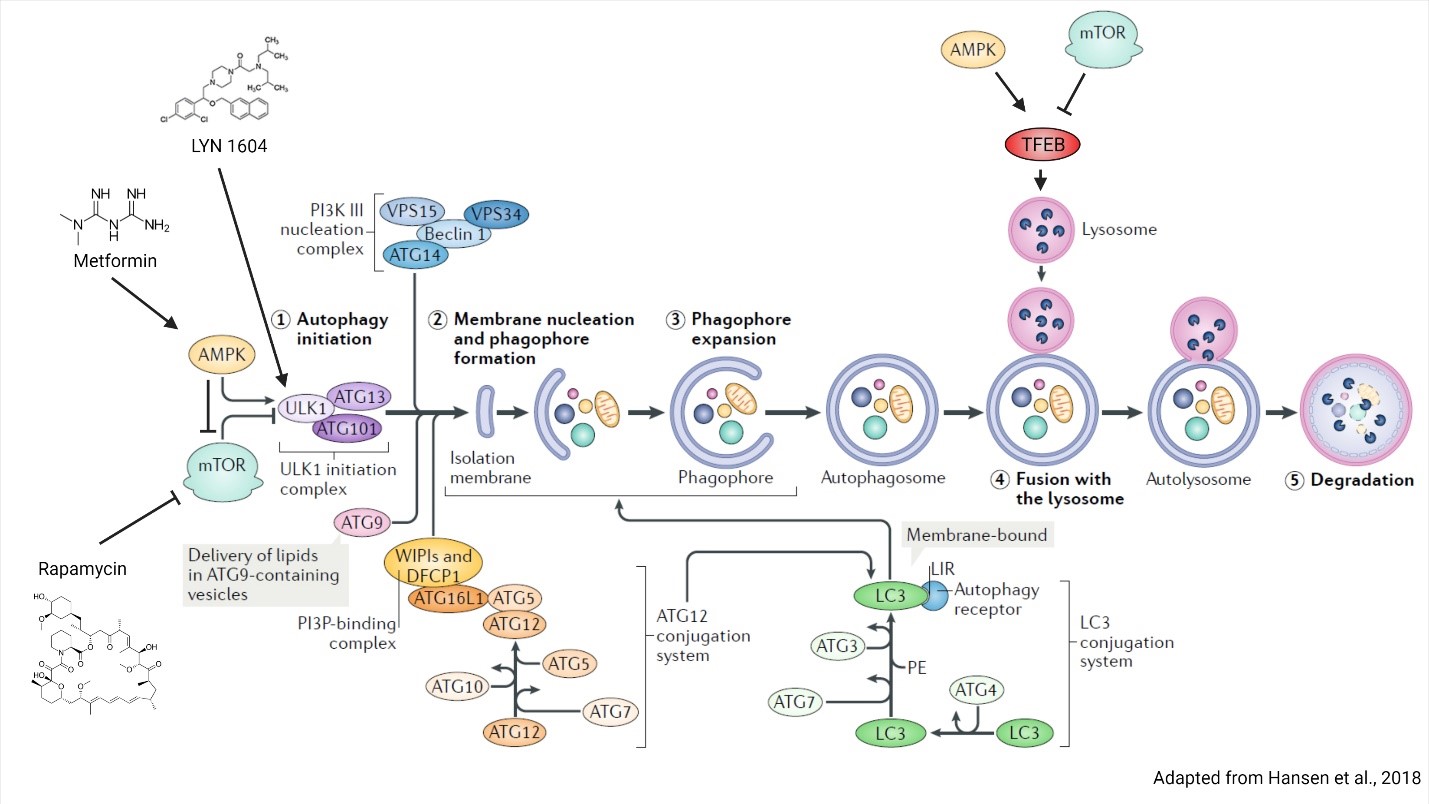
Figure 2. Regulation of autophagy by AMPK and mTOR. AMPK and mTOR directly regulate autophagy through regulating phagophore and lysosome biogenesis. Phagophore formation is regulated through ULK1 complex phosphorylation, whereas lysosome biogenesis is controlled by TFEB transcriptional activity. Autophagy inducing compounds rapamycin, metformin and LYN 1604 and their protein targets are indicated. Figure is adapted from Hansen et al., 2018.
References
Blagosklonny, M. V. (2019). Rapamycin for longevity: Opinion article. Aging, 11(19), 8048–8067. https://doi.org/10.18632/aging.102355
Dumont, N. A., Wang, Y. X., Von Maltzahn, J., Pasut, A., Bentzinger, C. F., Brun, C. E., & Rudnicki, M. A. (2015). Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nature Medicine, 21(12), 1455–1463. https://doi.org/10.1038/nm.3990
García-Prat, L., Martínez-Vicente, M., Perdiguero, E., Ortet, L., Rodríguez-Ubreva, J., Rebollo, E., Ruiz-Bonilla, V., Gutarra, S., Ballestar, E., Serrano, A. L., Sandri, M., & Muñoz-Cánoves, P. (2016). Autophagy maintains stemness by preventing senescence. Nature, 529(7584), 37–42. https://doi.org/10.1038/nature16187
Hansen, M., Rubinsztein, D. C., & Walker, D. W. (2018). Autophagy as a promoter of longevity: insights from model organisms. Nature Reviews Molecular Cell Biology, 19(9), 579–593. https://doi.org/10.1038/s41580-018-0033-y
Higashi, T., Friedman, S. L., & Hoshida, Y. (2017). Hepatic stellate cells as key target in liver fibrosis. Advanced Drug Delivery Reviews, 121, 27–42. https://doi.org/10.1016/J.ADDR.2017.05.007
Huang, Q., Liu, Y., Zhang, S., Yap, Y. T., Li, W., Zhang, D., Gardner, A., Zhang, L., Song, S., Hess, R. A., & Zhang, Z. (2020). Autophagy core protein ATG5 is required for elongating spermatid development, sperm individualization and normal fertility in male mice. Autophagy, 1–15. https://doi.org/10.1080/15548627.2020.1783822/SUPPL_FILE/KAUP_A_1783822_SM7639.ZIP
Joshi, A., & Kundu, M. (2013). Mitophagy in hematopoietic stem cells: The case for exploration. In Autophagy (Vol. 9, Issue 11, pp. 1737–1749). https://doi.org/10.4161/auto.26681
Jung, C. H., Ro, S. H., Cao, J., Otto, N. M., & Kim, D. H. (2010). MTOR regulation of autophagy. In FEBS Letters (Vol. 584, Issue 7, pp. 1287–1295). https://doi.org/10.1016/j.febslet.2010.01.017
Kim, J., Yang, G., Kim, Y., Kim, J., & Ha, J. (2016). AMPK activators: mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224. https://doi.org/10.1038/EMM.2016.16
Lampert, M. A., Orogo, A. M., Najor, R. H., Hammerling, B. C., Leon, L. J., Wang, B. J., Kim, T., Sussman, M. A., & Gustafsson, Å. B. (2019). BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation) BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor c. Autophagy, 15(7), 1182–1198. https://doi.org/10.1080/15548627.2019.1580095
Li, J., Zeng, C., Zheng, B., Liu, C., Tang, M., Jiang, Y., Chang, Y., Song, W., Wang, Y., & Yang, C. (2018). HMGB1-induced autophagy facilitates hepatic stellate cells activation: A new pathway in liver fibrosis. Clinical Science, 132(15), 1645–1667. https://doi.org/10.1042/CS20180177
Mirzaei, H., Sahebkar, A., Sichani, L. S., Moridikia, A., Nazari, S., Nahand, J. S., Salehi, H., Stenvang, J., Masoudifar, A., Mirzaei, H. R., & Jaafari, M. R. (2018). Therapeutic application of multipotent stem cells. Journal of Cellular Physiology, 233(4), 2815–2823. https://doi.org/10.1002/JCP.25990/FORMAT/PDF
Palma, C. De, Morisi, F., Cheli, S., Pambianco, S., Cappello, V., Vezzoli, M., Rovere-Querini, P., Moggio, M., Ripolone, M., Francolini, M., Sandri, M., & Clementi, E. (2012). Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death & Disease, 3(11), e418. https://doi.org/10.1038/CDDIS.2012.159
Parzych, K. R., & Klionsky, D. J. (2014). An overview of autophagy: Morphology, mechanism, and regulation. In Antioxidants and Redox Signaling (Vol. 20, Issue 3, pp. 460–473). Mary Ann Liebert, Inc. https://doi.org/10.1089/ars.2013.5371
Raben, N., & Puertollano, R. (2016). TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress. In Annual Review of Cell and Developmental Biology (Vol. 32, pp. 255–278). Annual Reviews. https://doi.org/10.1146/annurev-cellbio-111315-125407
Sousa-Victor, P., Gutarra, S., García-Prat, L., Rodriguez-Ubreva, J., Ortet, L., Ruiz-Bonilla, V., Jardí, M., Ballestar, E., González, S., Serrano, A. L., Perdiguero, E., & Muñoz-Cánoves, P. (2014). Geriatric muscle stem cells switch reversible quiescence into senescence. Nature, 506(7488), 316–321. https://doi.org/10.1038/nature13013
Tang, A. H., & Rando, T. A. (2014). Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. The EMBO Journal, 33(23), 2782–2797. https://doi.org/10.15252/embj.201488278
Thoen, L. F. R., Guimarães, E. L. M., Dollé, L., Mannaerts, I., Najimi, M., Sokal, E., & Van Grunsven, L. A. (2011). A role for autophagy during hepatic stellate cell activation. Journal of Hepatology, 55(6), 1353–1360. https://doi.org/10.1016/j.jhep.2011.07.010
Wirawan, E., Vanden Berghe, T., Lippens, S., Agostinis, P., & Vandenabeele, P. (2011). Autophagy: for better or for worse. Cell Research, 22, 43–61. https://doi.org/10.1038/cr.2011.152
Xiang, H., Zhang, J., Lin, C., Zhang, L., Liu, B., & Ouyang, L. (2020). Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharmaceutica Sinica B, 10(4), 569–581. https://doi.org/10.1016/J.APSB.2019.10.003
Yuan, Y., Fang, Y., Zhu, L., Gu, Y., Li, L., Qian, J., Zhao, R., Zhang, P., Li, J., Zhang, H., Yuan, N., Zhang, S., Ma, Q., Wang, J., & Xu, Y. (2020). Deterioration of hematopoietic autophagy is linked to osteoporosis. Aging Cell, 19(5). https://doi.org/10.1111/acel.13114
Zhang, J., Jiang, N., Ping, J., & Xu, L. (2021). TGF-β1-induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. International Journal of Molecular Medicine, 47(1), 256–266. https://doi.org/10.3892/ijmm.2020.4778
Zhou, Y., Zhang, Q., Kong, Y., Guo, X., Zhang, H., Fan, H., & Liu, L. (2020). Insulin-Like Growth Factor Binding Protein-Related Protein 1 Activates Primary Hepatic Stellate Cells via Autophagy Regulated by the PI3K/Akt/mTOR Signaling Pathway. Digestive Diseases and Sciences, 65(2), 509–523. https://doi.org/10.1007/s10620-019-05798-x
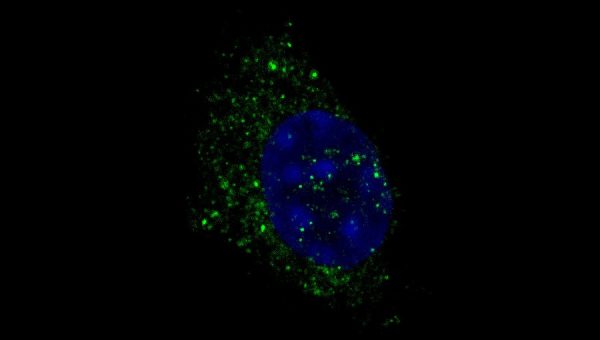
Image: Immunofluorescence of autophagosome structures in muscle stem cell-derived primary myoblasts. Immunostaining was performed with WIPI2 antibody (green) and cells were counterstained with DAPI (blue).
