MRM Insights: IPSC-Derived Oligodendrocytes – a model for regenerative potential in MS?

Valerio Piscopo

Thomas Durcan

Gabriela Blaszczyk

Jack Antel
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Jack Antel, Professor of Neurology and Neurosurgery at the Neuro and McGill University, Gabriela Blaszczyk, Undergraduate student in his lab, Thomas Durcan, Associate Professor at The Neuro and McGill University, Director of the EDDU, and Valerio Piscopo, Research Associate at the EDDU, are discussing iPSC-Derived Oligodendrocytes as a model for regenerative potential in Multiple Sclerosis.
IPSC-Derived Oligodendrocytes – a model for regenerative potential in Multiple Sclerosis?
Oligodendrocytes (OLs) are a population of glial cells whose myelin processes ensheath axons, permitting efficient signal transduction within the central nervous system (CNS). The OLs provide essential trophic support to neurons. The pathologic hallmark of multiple sclerosis (MS) is myelin destruction. The initial phase of the MS disease course is characterized by recurrent relapses of neurologic dysfunction resulting from focal inflammation-associated demyelinating lesions with relative sparing of OL cell bodies (1). Recovery from relapses relates at least in part to remyelination within such lesions. Over time the disease can evolve towards a progressive phase with gradual worsening of the neurological symptoms; this phase is associated with loss of OL cell bodies, with the inability to regenerate the myelin sheath and subsequent neuronal loss (1). Remyelination within the CNS is usually attributed to recruitment and differentiation of oligodendrocyte progenitor cells (OPCs) (2), although previously myelinating OLs may also contribute.
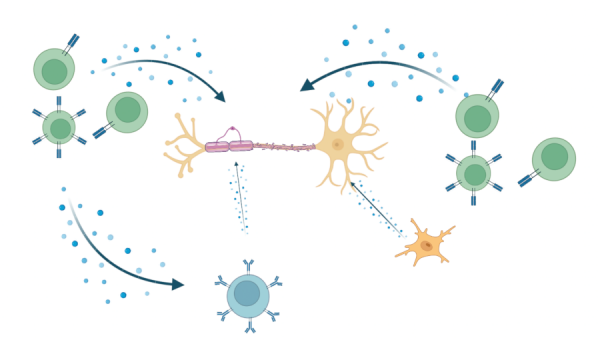
The precise mechanisms underlying the injury and repair processes in MS remain to be fully defined. Implicated as mediators in the injury processes are the constituents of inflammation that can be derived from infiltrating immune cells, as well as from activated astrocytes and microglia (Fig 1). Conditions within the microenvironment of MS lesions create metabolic stress on the OLs. Success in myelin repair has been linked to production of essential trophic factors and overcoming local inhibitory conditions. Animal models, such as experimental autoimmune encephalomyelitis (EAE) and toxin-induced demyelination, have been developed to assess both the injury and repair processes associated with MS.
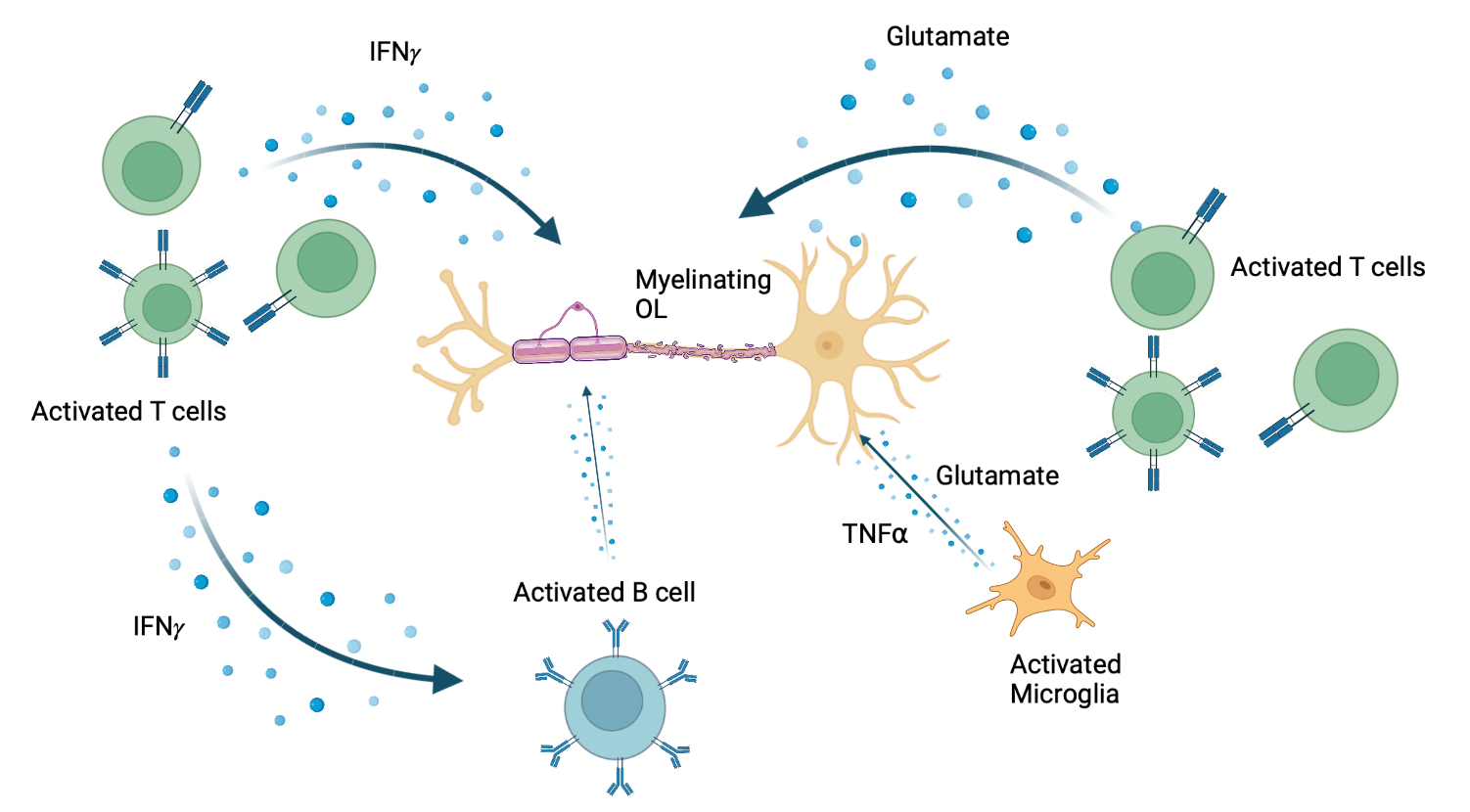
Figure 1. Release of pro-inflammatory molecules which act on OLs during MS lesion formation. Made with BioRender. Adapted from: Wu GF, Alvarez E. Neurol Clin. 2011. Levite M. J Neural Transm (Vienna). 2017. Frigo M, et al. Curr Med Chem. 2012
An ongoing challenge is to demonstrate the specific molecular features of human OLs that underlie their susceptibility to injury and their capacity to carry out repair. Direct analysis of MS affected tissues provides guidance but is limited with regard to defining the dynamic aspects of the injury/repair processes. Access to primary human OLs for functional and molecular studies is also limited although conducted in our program through the collaborative efforts of the McGill adult and pediatric neurosurgical departments at McGill, and the Neuroimmunology program at the MNI (3).
An emerging approach to advance our understanding of the injury /repair properties of human OLs involves the use of human-induced pluripotent stem cells (iPSCs), as they can be easily derived from somatic cells of patients and can differentiate into all cell types (4). This approach is used by the collaboration of the MNIs Neuroimmunology Program, the Neuro’s Open Science program through the C-BIG repository (https://www.mcgill.ca/neuro/open-science/c-big-repository) and the Early Drug Development Unit (EDDU) with support from the MRM. Using a previously established protocol (5), we can effectively generate OPCs, allowing us to study their susceptibility to injury and potential mechanisms contributing to their deficiency in repair under conditions as seen in MS.
We have used the iPSC-derived OPCs to advance studies as to whether and how pro-inflammatory molecules contribute to the demise of OLs and their precursors. Amongst such molecules, as illustrated in Figure 1 are glutamate that can be released by activated Th17 cells or resident microglia; interferon gamma (IFNγ), which can also be released by activated T cells, and tumour necrosis factor alpha (TNFα) produced by activated macrophages, T lymphocytes, and natural killer (NK) cells (6 – 8). Until now, the effects of these molecules have been explored in vivo in experimental models, in vitro using non-human OL lineage cells, and in our own in vitro studies using mature human OLs (9). The latter studies indicate that mediators induce process retraction but not cell death in the human cells, contrasting with observations made with non-human progenitor cells. The iPSC studies allow us to assess the effects of these mediators along the full spectrum of human OL cell differentiation. Using this approach, we observe that IFNγ and TNFα individually, arrest the differentiation of OPCs towards oligodendrocytes, rather than initiating cell death programs, a finding in agreement with Staros et al. who used a genetic re-programming approach to generate iPSC derived OLs (10). This assay system allows the evaluation of a wide range of molecules derived from immune (including B cells) (11) and glial cells.
Tracking developmental stage of OPCs in vitro requires intermittent complex immunocytochemical analyses. To enable us to track such cells dynamically over time, the MRM has specifically supported our efforts to generate an iPSC cell line expressing an OL lineage reporter gene (SOX10), allowing us to track the developmental differentiation of stem cells into and along the OL cell pathway (Fig. 2A). This was done at the EDDU by Zhipeng You and Valerio Piscopo using CRISPR/Cas-9 technology. These cells will now be sorted (Fig. 2B) and added to special plates containing nanofibers in order to follow their myelination potential (Fig. 2C).
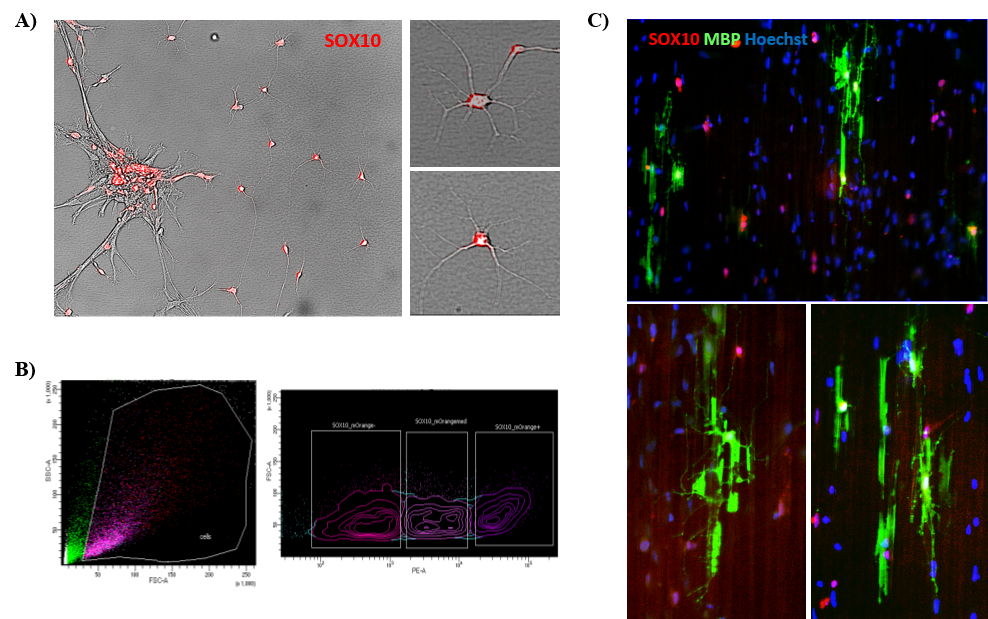
Figure 2. Production of SOX10-mOrange reporter iPSC line. A) Microscope image of SOX10-positive OPCs, showing the red fluorescence; B) The cells are live-sorted for the expression of the reporter and C) plated onto nanofiber plates to assess myelination, represented in green by the expression of the Myelin Basic Protein (MBP).
References
1. Heß K, Starost LJ, Kieran NW, Thomas C, Vincenten MCJ, Antel J, Martino G, Huitinga I, Healy L, Kuhlmann T. Lesion stage dependent causes for impaired remyelination in MS. Acta Neuropathol- 2020 Jul 24.
2. Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839-855. doi:10.1038/nrn2480
3. Luo JXX, Cui QL, Yaqubi M, Hall JA, Dudley R, Srour M, Addour N, Jamann H, Larochelle C, Blain M, Healy LM, Stratton JA, Sonnen JA, Kennedy TE, Antel JP. Human oligodendrocyte myelination potential; relation to age and differentiation. Ann Neurol. 2022 Feb;91(2):178-191. doi: 10.1002/ana.26288.Epub 2022 Jan 10.
4. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872. doi:10.1016/j.cell.2007.11.019
5. Douvaras P, Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10(8):1143-1154. doi:10.1038/nprot.2015.075
6. Larochelle C , Wasser B, Jamann H, Löffel JT, Cui QL, Tastet O, Stratton J, Schillner M, Luchtman D, Birkenstock J, Stroh A, Antel J, Bittner S, and Zipp F. Pro-inflammatory T-helper 17 directly harm oligodendrocytes in neuroinflammation. Proc Natl Acad Sci- 2021 Aug 24;118(34): e2025813118
7. Jamann H, Cui Q, Desu HL, Pernin F, Tastet O, Halaweh A, Farzam-kia N, Mamane VH, Ouédraogo O, Cleret-Buhot A, Daigneault A, Lemaitre F, Arbour N, Antel J, Stratton J, Larochelle C – Contact-dependent granzyme B-mediated cytotoxicity of Th17 cells towards human oligodendrocytes – Frontiers Immunology – in press
8. 8.Moore CS, fi Q, Warsi NM, Durafourt BA, Zorko N, Owen DR, Antel JP, Bar-Or A. Direct and Indirect Effects of Immune and Central Nervous System-Resident Cells on Human Oligodendrocyte Progenitor Cell Differentiation. J Immunol. 2015 Jan 15;194(2):761-72.
9. Pernin F, JXX, Cui QL, Blain M, Fernandes MGF, Yaqubi M, Srour M, Hall J, Dudley R, Jamann H, Larochelle C, Zandee SEJ, Prat A, Stratton J, Kennedy TEK, Antel JP. Diverse injury responses of human oligodendrocyte to mediators implicated in multiple sclerosis -BRAIN – in press
10. Starost L, Lindner M, Herold M, Xu YKT, Drexler HCA, Heß K, Ehrlich M, Ottoboni L, Ruffini F, Stehling M, Röpke A, Thomas C, Schöler HR, Antel J, Winkler J, Martino G, Klotz L, Kuhlmann T Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 2020 Sep 7.
11. Lisak RP, Benjamins JA, Nedelkoska L, Barger JL, Ragheb S, Fan B, Ouamara N, Johnson TA, Rajasekharan S, Bar-Or A. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J Neuroimmunol. 2012 May 15;246(1-2):85-95.
