MRM Insights: Effective clinical translation of stem cells – from bench, to clinic, to bench

Sangeeth Pillai

Jose Gil Munguia-Lopez

Simon Tran

Mari Kaartinen
Every month, in MRM Insights, a member of the MRM Network is writing about stem cells and regenerative medicine from a different perspective. This month, Associate Professor Mari Kaartinen and Professor Simon Tran, from McGill Faculty of Dental Medicine and Oral Health Sciences, and their trainees Jose Gil Munguia-Lopez, Postdoctoral Researcher, and Ph.D. Candidate Sangeeth Pillai, are discussing effective clinical translation of stem cells.
Effective clinical translation of stem cells – from bench, to clinic, to bench
‘Stem cells’ (SCs) is an umbrella term that includes several cell types that can self-renew and differentiate with a potential to be used for therapeutic applications1. Based on their clinical use and therapeutic purpose, SCs can be categorized into either ‘first generation’ multipotent somatic SCs such as hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) or ‘second generation’ pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). The first-generation SCs are lineage-restricted and give rise to tissue-specific cell types. In contrast, the second-generation SCs are pluripotent and can give rise to any cell type in the body2. The combination of the first- and second-generation SCs constitutes the core of innovative SC therapy. HSCs are the most common and widely used SCs clinically, and thousands of HSCs-based bone marrow transplants are performed yearly worldwide3. MSCs derived from several tissue sources such as bone marrow, adipose, umbilical cord, dental, and periodontal tissues are being tested in numerous clinical studies across the world4. PSCs-based therapies have been demonstrated to be safe and host-tolerant in phase 1 clinical trials5,6. However, ESC- and iPSC-based therapies pose several challenges such as obtaining clinical grade PSC products, threat of immune rejection and tumorigenicity, phenotypic heterogeneity, ethical concerns, etc.
In a nutshell, the pathway to take a SC therapy from the lab bench to the clinic is via pre-clinical studies, such as testing on cells ex vivo and on animals. If these approaches are successful, therapies can be tested on humans in a clinical trial7,8. Phase 1 trials test the safety and dosage of SC therapies in a small group of people. Phase 2 trials aim to test the efficacy, side effects, and to figure the best dose. Phase 3 trials test the efficacy and monitor side effects of the SC therapy on a large group of patients, including comparing the SC therapy to a commonly used treatment. If the phase 3 trial is successful, the regulatory agency (such as Health Canada or FDA) will allow the SC therapy to be used on the market. Phase 4 trials are done once the SC therapy is on the market to monitor its effectiveness and side effects in a larger population and over a longer period of time8.
From the NIH clinicaltrials.gov database, Trounson et al., in 20119 reported 123 clinical trials using MSC for various therapeutic applications. This search was repeated by the same researchers in 2015 and found a threefold increase with 374 registered trials using MSCs10. We recently renewed this search using the NIH database with the search term ‘Mesenchymal Stem Cells’ and found >1300 registered interventional trials reporting the use of MSC. Among these, 1112 clinical trials were categorized into either early phase 1, phase 1 and or phase 2 trials, 88 studies were reported to be in phase 3, and surprisingly only 8 clinical trials were reported in phase 4. These statistics point towards an alarming rise in MSC-based studies that are registered annually with very limited translation outside the clinical pipeline, and notably, many of these trials are small-scale studies with varying designs, making it challenging to perform an unbiased analysis of the outcomes11. In addition to these trials, there are several other unregulated treatments and stem cell products that are made available directly to consumers without the approval from regulatory agencies12.
What might be the reason for this loss in translation within the clinical pipeline? Undoubtedly, stem cells have a wide spectrum of implications in advancing innovativeness in healthcare, either as cell therapy candidates (cell extracts and paracrine factors) or as tool kits in disease modelling and drug discovery (organoids). However, this innovative translation is mostly fraught by our partial knowledge, lack of adequate technology, and sometimes lack of understanding of the rules that govern the stemness of the cells. In 2006, the International Society of Cellular Therapy (ISCT) recommended criteria to define multipotent MSCs and provided basic guidelines on the minimal characterization of MSCs13. A recent analysis of 84 published clinical trials on MSC characterization data revealed that: the majority of these trials did not provide any data on the differentiation potential of MSC, 28 studies reported no data on cell characterization, 45 studies reported the average value of cell markers from a few lots of cells used in the trials, and only 6 papers defined their functionality which meant that there was no significant justification for the intended mechanism of action of the cells for the condition being treated14. Labelling the MSCs as therapeutic agents without definitive evidence of their paracrine or immunomodulatory potential, in this context, even the fundamental evidence, is misrepresentative. The SC niche is multifarious and so to efficiently utilize their therapeutic capacities, several strategies should be employed. Moreover, these strategies and tools (in vitro/in vivo studies) should be devised to appropriately evaluate SCs at different complexities such as, at a single SC level, a population of SC and their presence in complex tissues and organ systems. However, only limited research groups are equipped with such a wide range of resources, archetypal disease models, and expertise to deconvolute the highly dynamic, heterogenous nature of SC systems for effective clinical translation. All these barriers impede the current and long-term clinical success of SC therapy and often lead to premature commercialization.
Earlier this year, the Kaartinen and Tran research teams from the Faculty of Dental Medicine and Oral Health Sciences, along with the Center for Bone and Periodontal Research (CBPR) core facility at McGill University, applied for a Canada Foundation of Innovation (CFI) infrastructure grant to create a new research pipeline to harness and test the regenerative potential of bone and periodontal stem cells and their therapeutic modulation. The idea of the proposed infrastructure is to support our innovative research programs and multiple projects aimed at the isolation, expansion, and modification (ex vivo and in vivo) of SCs, followed by their transplantation, for tissue engineering, and regenerative applications to improve bone, periodontal, and oral health. Our research programs also include translating our newly generated knowledge on mechanisms of stem cell differentiation and behaviour to the discovery and testing of novel targets for therapeutical purposes.
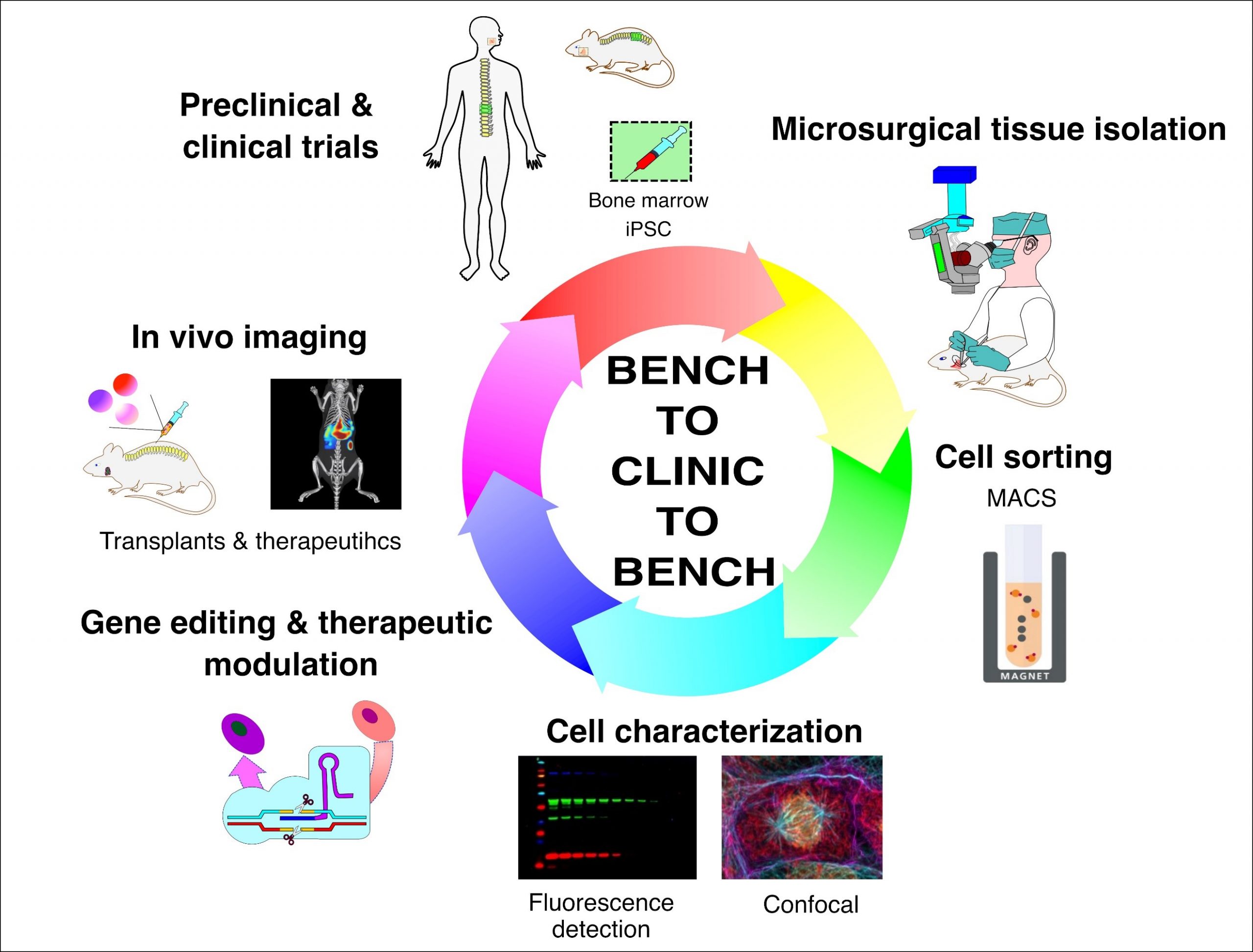
Our proposed bench-to-clinic-to-bench infrastructure pipeline for stem cell isolation, sorting, characterization, transplantation, and tracking using the current state-of-the-art technologies.
We divide our research projects into three specific arms to achieve our objectives with this proposed infrastructure. First, the tissue engineering arm that focuses on developing ex-vivo, in vitro, and in vivo soft (oral) and hard (bone) tissue models, including disease models. The proposed strategy to use SC to develop ex vivo models and as cell therapy agents will utilize the requested microsurgical instruments to precisely dissect, identify, and isolate oral and bone marrow stem cells. Any SC research aiming at future clinical translation must implement stringent cell purification strategies to validate the SCs before preclinical testing. To fulfill this criterion, we proposed the use of an automated and highly versatile magnetic cell sorting system. Second, the regenerative arm centers on identifying and isolating key active biological factors/proteins from the human and mice craniofacial tissue and bone marrow-derived MSCs, including labial and periodontal SCs. To fulfil our goal of harnessing the SCs’ regenerative potential as a therapeutic agent and after organoid development, we aim at their biological and functional characterization with new generation, multi-mode fluorescence imager and a confocal microscope for both in vitro tests and after re-implantation in vivo. Third, the therapeutic arm aims to modulate SCs via ex vivo gene editing and in vivo novel therapeutics targeting stem cell differentiation. The core instrument requested in our pipeline is the highly sensitive live-animal imaging system that will allow tracking of transplanted SCs, biomaterials as well as injected in vivo therapeutics in our rodent models which will play a critical role in the success/endpoint evaluation of preclinical trials. This in vivo analysis will allow multiple sequential measurements in the same animal significantly reducing the expenses and animals used, thereby accelerating research projects and discovery. Moreover, the pipeline’s equipment will use near-infrared (NIR) light detection and imaging. The deep penetration of NIR light (wavelengths (600-1900 nm) decreases autofluorescence that is common at visible wavelengths, and reduces scattering which all increase resolution and detection sensitivity. All of the instruments requested in this grant application are compatible with each other and will fulfil a certain niche in SC research.
Our ultimate goal is to utilize the currently available and hopefully the upcoming resources to devise strategies that can improve our knowledge of the mechanisms that dictate the use of SCs in preclinical and clinical research. We hope this infrastructure pipeline will also support the creation of innovative translational projects within our McGill research community, such as the McGill Regenerative Medicine (MRM) Network, harbouring new collaborations aiming at SC therapies and SC modulation in a BENCH to CLINIC to BENCH manner.
References
1. Atala, A., Lanza, R., Mikos, T. & Nerem, R. Principles of regenerative medicine. (Academic press, 2018).
2. Williams, L. A., Davis-Dusenbery, B. N. & Eggan, K. C. SnapShot: directed differentiation of pluripotent stem cells. Cell 149, 1174-1174. e1171 (2012).
3. Kimbrel, E. A. & Lanza, R. Next-generation stem cells—ushering in a new era of cell-based therapies. Nature Reviews Drug Discovery 19, 463-479 (2020).
4. Hass, R., Kasper, C., Böhm, S. & Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling 9, 1-14 (2011).
5. Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. The Lancet 379, 713-720 (2012).
6. Mandai, M. et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. New England Journal of Medicine 376, 1038-1046 (2017).
7. Clinical trials and drug safety, <https://www.canada.ca/en/health-canada/services/clinical-trials.html> (2022).
8. Clinical Research, <https://www.fda.gov/patients/drug-development-process/step-3-clinical-research> (2022).
9. Trounson, A., Thakar, R. G., Lomax, G. & Gibbons, D. Clinical trials for stem cell therapies. BMC medicine 9, 1-7 (2011).
10. Trounson, A. & McDonald, C. Stem cell therapies in clinical trials: progress and challenges. Cell stem cell 17, 11-22 (2015).
11. Martin, I., Galipeau, J., Kessler, C., Le Blanc, K. & Dazzi, F. Challenges for mesenchymal stromal cell therapies. Science translational medicine 11, eaat2189 (2019).
12. Sipp, D., Robey, P. G. & Turner, L. (Nature Publishing Group, 2018).
13. Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315-317 (2006).
14. Wilson, A. J., Rand, E., Webster, A. J. & Genever, P. G. Characterisation of mesenchymal stromal cells in clinical trial reports: analysis of published descriptors. Stem cell research & therapy 12, 1-15 (2021).
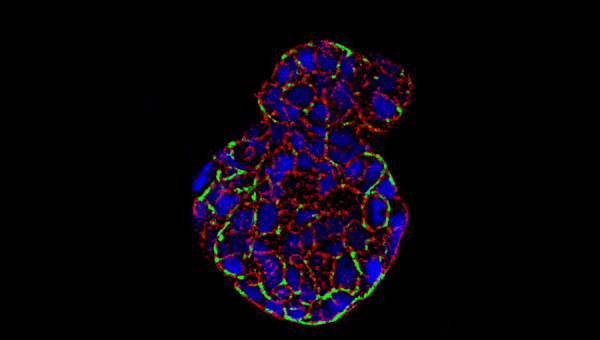
Photo Credit: Salivary gland spheroid stained with F-actin (red), high-intensity areas (green), and nuclei (blue), courtesy of Jose Gil Munguia-Lopez and Sangeeth Pillai.
