MRM Insights: Regenerating the Native Microenvironment with Decellularized Extracellular Matrix

Nicole Li-Jessen

Mika Brown
Every month, in the MRM Insights, a member of the MRM Network writes about stem cells and regenerative medicine from a different perspective. This month, Mika Brown, Ph.D. candidate in the Department of Biological and Biomedical Engineering, and her supervisor Nicole Li-Jessen, Associate Professor in the School of Communication Sciences and Disorders, are discussing how to regenerate the native microenvironment with decellularized extracellular matrix.
Regenerating the Native Microenvironment with Decellularized Extracellular Matrix – Toward Lasting Treatments
Decellularized extracellular matrix (dECM) is a natural biomaterial that is derived from human or animal tissue by removing the cells and acellular antigens.[1] One great advantage of dECM is that it provides a natural environment that cells are already familiar with and can function in effectively. dECM materials have shown excellent regenerative potential by minimizing the foreign body response, stimulating vascularization, and promoting ECM remodeling.[2] Essentially, dECM contains structural proteins (e.g. collagens and elastin), macromolecules (e.g. proteoglycans and glycosaminoglycans) that play complex roles in tissue homeostasis and repair.[1] Matrix-bound small molecules such as growth factors and particles such as extracellular vesicles may also be retained following decellularization.[1, 3] Growth factors stimulate regenerative processes including angiogenesis while extracellular vesicles contribute to immune cell modulation and homeostasis. Thanks to its complex structure and composition, dECM has a unique ability to stimulate native wound healing processes ranging from cellular recruitment, stem cell differentiation, immune modulation to neo-ECM production.
The Current State of dECM Biomaterial Research
In the past, dECM biomaterials were primarily fabricated as whole decellularized organs or sheets.[1, 4] These dECM biomaterials could be recellularized for intended applications in lung or aortic transplants or, more successfully, used as acellular soft tissue grafts. However, the physicochemical properties of whole dECM biomaterials are impacted by the decellularization process, resulting in structural and mechanical changes, inefficient cell-matrix interactions, and suboptimal outcomes.[5, 6]
Current dECM biomaterial research has evolved toward biomaterials constructed from dECM comminuted into small particles, solubilized with enzymes, and recapitulated into different forms such as hydrogels.
We recently published a comprehensive review paper on current trends in dECM regenerative biomaterials.[1] In our review, dECM-particle based biomaterials has grown exponentially in the past decade (2012 – 2021), from only 7 archival papers in 5 tissue types to 93 papers over 19 tissue types. The types of dECM biomaterials have also expanded from injectable hydrogels, molded scaffolds, and electrospun scaffolds to include bioprinted scaffolds and aerosolized nanoparticles. In this article, we focus on discussing injectable hydrogels, electrospun scaffolds, and bioprinted scaffolds with some classical examples (Figure 1).
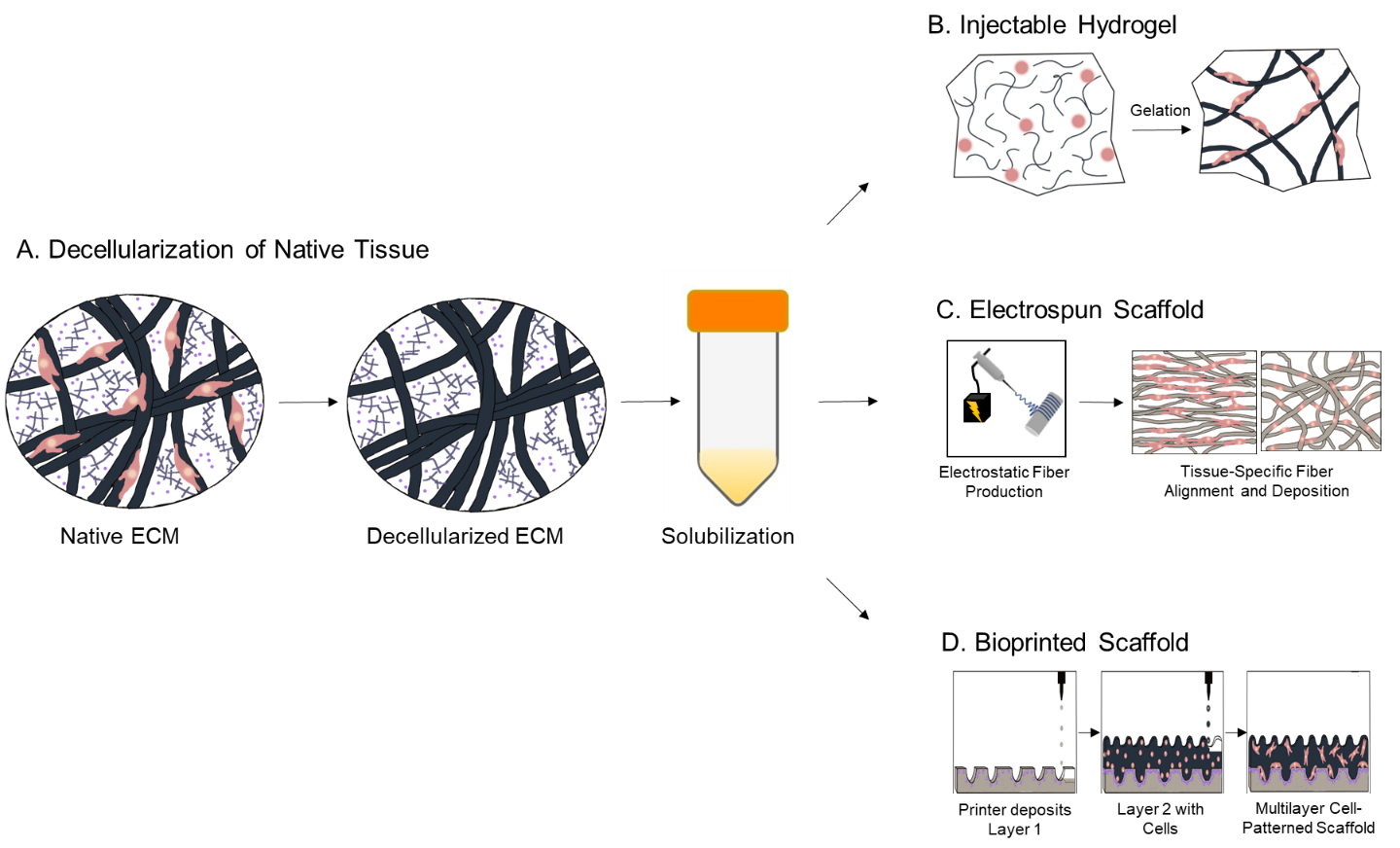
Figure 1. Decellularization and Fabrication of Three Promising Candidates for dECM Particle-Based Biomaterials. A. Decellularization and solubilization of dECM. B. Formation of injectable hydrogels. C. Deposition of fibers in electrospun scaffolds. D. Patterning of multilayer, cell-laden bioprinted scaffolds.[1]
Leading Types of dECM Biomaterials
Injectable dECM Hydrogels are prepared by solubilizing dECM particles, after which a hydrogel forms when incubated at 37℃, or physiological temperature.[1] Hydrogels are the most common dECM biomaterial because they can be injected to completely fill defects, and have low costs of labor and production for scale-up. One notable recent dECM hydrogel was prepared by crosslinking lung dECM with photopolymerizable poly(ethylene glycol) (PEG) using thiol-ene click chemistry.[7] In this study, photopolymerization was performed after seeding with fibroblasts to induce the hydrogels to stiffen from an elastic modulus characteristic of a healthy lung to that of a fibrotic lung. Researchers studied how the stiffened environment affected the production of the fibrotic proteins Collagen 1a1 and α-Smooth Muscle Actin by lung fibroblasts. By day 7, expression of both fibrotic proteins was up to 3-fold higher than unstiffened hydrogel controls.
Electrospun scaffolds are prepared by drawing microfibers through a metallic needle containing solubilized dECM solution using high voltage.[1] The microfibers are deposited onto a collector, and layered to produce a scaffold. Electrospun scaffolds have the ability to mimic tissue architecture by creating a pattern of fibers that can be tailored for specific applications, such as a concentric radial distribution for corneal repair, renal filtration membrane for kidneys, or a tube-shaped bridge for axon regeneration.[8-10] In a corneal dECM study, the radial distribution of dECM-polycaprolactone (PCL) fibers aided corneal stromal keratinocytes in bridging between fibers and adopting their native morphology compared to a scaffold with randomly aligned fibers.[8]
dECM bioink is printed by following a pattern drop by drop to replicate the dimensions and structure of a defect, tissue, or organ, forming a bioprinted scaffold.[1] Bioprinting is promising for the replication of structures with complex or layered tissue architecture, and locoregionally varied mechanical properties. Cells can be printed together with the bioink in specific regions of the scaffold to aid in the development of complex structures, such as cholangiocyte-laden bile ducts in the liver.[11] For instance, a “wrinkled” cylindrical PCL frame containing bioprinted layers of esophageal muscle dECM with smooth muscle cells and mucosal dECM with esophageal epithelial cells was designed to mimic a segment of the esophagus.[12] Compared to a collagen I-PCL control, dECM-PCL scaffolds exhibited significantly higher expression of α-smooth muscle actin by smooth muscle cells and E-cadherin by epithelial cells.
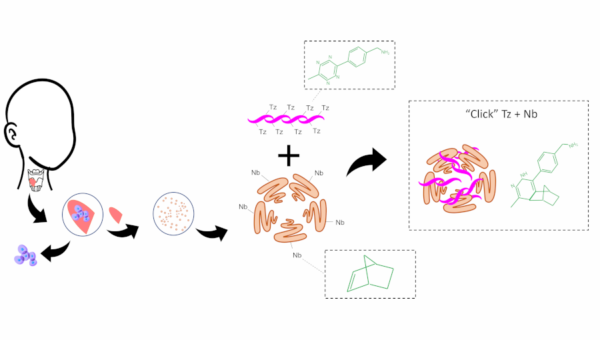
Click dECM Hydrogels for Permanent Vocal Fold Regeneration
The majority of the dECM materials discussed in this article are crosslinked composites. Crosslinking and composites can both aid in augmenting the mechanical properties and stability of natural materials and reducing scaffold contraction by cells. For instance, the dECM-PEG and dECM-PCL scaffolds previously discussed all achieved mechanical properties equal or greater to the healthy target tissue.[7, 8, 12] Collagen scaffolds have been shown to resist contraction in cell culture using crosslinking methods such as methacrylation and gluteraldehyde.[13, 14] dECM scaffolds can also suffer from contraction in cell culture.[15] Click crosslinking with natural polymers (like alginate or chitosan) produces composite materials without toxic side products that remain biodegradable, but with slowed rates of degradation that better enable regeneration.[16]
My project at Prof. Nicole Li-Jessen’s Voice and Upper Airway Lab at McGill focuses on developing dECM biomaterials for the repair of soft tissue defects, particularly the vocal folds in the larynx. Adult human vocal folds are uniquely small organs with dimensions of 10-20 mm longitudinal length, 8-12 mm transversal length, and 3-10 mm thickness.[17] Injectable hydrogels are ideal for the treatment of vocal fold defects because they can be applied using minimally invasive procedures. However, current vocal fold injectables require periodic reinjection and do not provide permanent solutions for tissue regeneration.
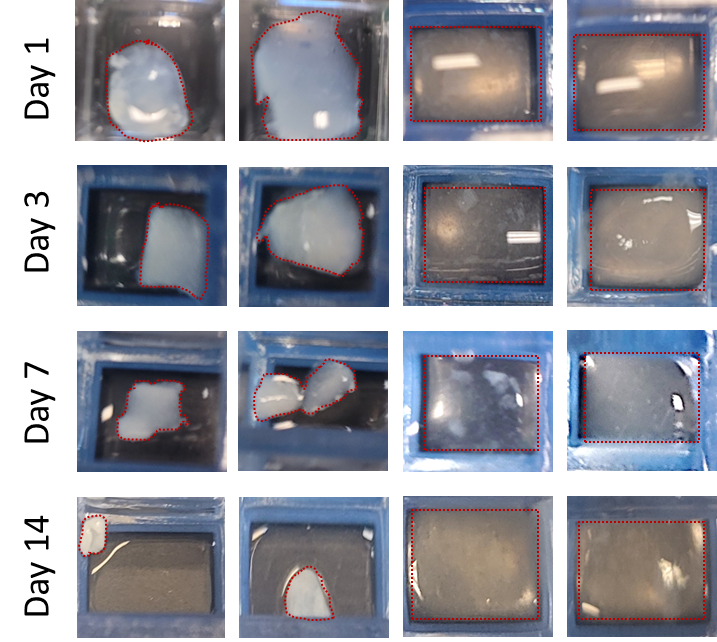
Figure 2. Contraction of Click dECM-Alginate and dECM-only Hydrogels over 14 Days. Click dECM-alginate hydrogels resist contraction while dECM-only Hydrogels showed up to 90% contraction.
A current trend in dECM biomaterials is to use the materials derived from the target tissue, considering that the ECM composition differs across tissues and contains tissue-specific cues.[1, 18] However, the small size of the vocal fold presents a challenge for scale-up of vocal fold dECM. I have completed a proteomic study comparing dECM from porcine vocal fold to commercially available porcine small intestinal submucosa (SIS) to determine whether an alternative soft tissue source may be used in vocal fold applications. SIS dECM fulfills the fabrication challenge of supply and accessibility while vocal fold dECM contains target tissue-specific bioactive molecules. We found that vocal fold dECM possesses greater similarity to native vocal fold than SIS dECM, pointing toward tissue-specific effects. However, SIS dECM stimulated the production of key vocal fold ECM components, elastin and HA, at comparable levels to vocal fold dECM with less in-group variation. These results indicated that SIS dECM may prove a viable, scalable alternative to tissue-specific vocal dECM.
In addition, to overcome the need for reinjection due to rapid biomaterial degradation, I am developing an injectable hydrogel produced from click dECM and alginate. Both vocal fold and SIS dECM are used in this study. Both click dECM-alginate hydrogels possess greater mechanical and structural stability than dECM-only hydrogels. For example, the Young’s modulus of the click dECM-alginate hydrogels (0.5-3 kPa) is within the range of the native VF (0.5-13 kPa),[19] and significantly greater than dECM-only hydrogels (0.015-0.06 kPa). While dECM-only hydrogels exhibit up to 90% contraction over 14 days, the click dECM-alginate hydrogels were shown to resist contraction from human vocal fold fibroblast cultures (Figure 2). Our ongoing work brings a more stable and scalable natural injectable biomaterial to the field of vocal fold tissue engineering, presenting one option for permanent vocal fold regeneration.
References
[1] M. Brown, J. Li, C. Moraes, M. Tabrizian, N.Y.K. Li-Jessen, Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine, Biomaterials 289 (2022) 121786.
[2] P.T. Coburn, X. Li, J. Li, Y. Kishimoto, N.Y. Li-Jessen, Progress in Vocal Fold Regenerative Biomaterials: An Immunological Perspective, Advanced NanoBiomed Research (2021) 2100119.
[3] L. Huleihel, G.S. Hussey, J.D. Naranjo, L. Zhang, J.L. Dziki, N.J. Turner, D.B. Stolz, S.F. Badylak, Matrix-bound nanovesicles within ECM bioscaffolds, J Science advances 2(6) (2016) e1600502.
[4] S.F. Badylak, P.V. Kochupura, I.S. Cohen, S.V. Doronin, A.E. Saltman, T.W. Gilbert, D.J. Kelly, R.A. Ignotz, G.R. Gaudette, The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium, Cell Transplantation 15(1_suppl) (2006) 29-40.
[5] L.T. Saldin, M.C. Cramer, S.S. Velankar, L.J. White, S.F. Badylak, Extracellular matrix hydrogels from decellularized tissues: Structure and function, Acta Biomaterialia 49 (2017) 1-15.
[6] T.J. Keane, I.T. Swinehart, S.F. Badylak, Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and In Vivo Relevance, Methods 84 (2015) 25-34.
[7] C.L. Petrou, T.J. D’Ovidio, D.A. Bölükbas, S. Tas, R.D. Brown, A. Allawzi, S. Lindstedt, E. Nozik-Grayck, K.R. Stenmark, D.E. Wagner, Clickable Decellularized Extracellular Matrix as a New Tool for Building Hybrid-Hydrogels to Model Chronic Fibrotic Diseases In Vitro, Journal of Materials Chemistry B 8(31) (2020) 6814-6826.
[8] J. Fernández-Pérez, K.E. Kador, A.P. Lynch, M. Ahearne, Characterization of extracellular matrix modified poly(ε-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration, Materials Science and Engineering C 108 (2020) 110415.
[9] S. Chen, Z. Du, J. Zou, S. Qiu, Z. Rao, S. Liu, X. Sun, Y. Xu, Q. Zhu, X. Liu, H.Q. Mao, Y. Bai, D. Quan, Promoting Neurite Growth and Schwann Cell Migration by the Harnessing Decellularized Nerve Matrix onto Nanofibrous Guidance, ACS Applied Material Interfaces 11(19) (2019) 17167-17176.
[10] R. Sobreiro-Almeida, D.R. Fonseca, N.M. Neves, Extracellular matrix electrospun membranes for mimicking natural renal filtration barriers, Materials Science and Engineering C Materials Biological Applications 103 (2019) 109866.
[11] P.L. Lewis, J. Su, M. Yan, F.Y. Meng, S.S. Glaser, G.D. Alpini, R.M. Green, B. Sosa-Pineda, R.N. Shah, Complex bile duct network formation within liver decellularized extracellular matrix hydrogels, Scientific Reports 8(1) (2018) 1-14.
[12] H. Nam, H.-J. Jeong, Y. Jo, J.Y. Lee, D.-H. Ha, J.H. Kim, J.H. Chung, Y.-S. Cho, D.-W. Cho, S.-J. Lee, J. Jang, Multi-layered Free-form 3D Cell-printed Tubular Construct with Decellularized Inner and Outer Esophageal Tissue-derived Bioinks, Scientific Reports 10(1) (2020) 7255.
[13] C. Lotz, F.F. Schmid, E. Oechsle, M.G. Monaghan, H. Walles, F. Groeber-Becker, Cross-linked Collagen Hydrogel Matrix Resisting Contraction To Facilitate Full-Thickness Skin Equivalents, ACS Applied Materials & Interfaces 9(24) (2017) 20417-20425.
[14] L. Dong, Q. Liu, Y. Gao, H. Jia, W. Dai, L. Guo, H. Fan, Y. Fan, X. Zhang, The effect of collagen hydrogels on chondrocyte behaviors through restricting the contraction of cell/hydrogel constructs, Regenerative Biomaterials 8(4) (2021) rbab030.
[15] M. Dabaghi, N. Saraei, M.B. Carpio, V. Nanduri, J. Ungureanu, M. Babi, A. Chandiramohan, A. Noble, S.D. Revill, B. Zhang, K. Ask, M. Kolb, Y. Shargall, J. Moran-Mirabal, J.A. Hirota, A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture, Cells 10(6) (2021) 1538.
[16] Y. Deng, A. Shavandi, O.V. Okoro, L. Nie, Alginate modification via click chemistry for biomedical applications, Carbohydrate Polymers 270 (2021) 118360.
[17] M. Hirano, Y. Kakita, K. Ohmaru, S. Kurita, Structure and Mechanical Properties of the Vocal Fold1 1A portion of this article was presented at the Vocal Fold Physiology Conference, Kurume, Japan, in January 1980, in: N.J. Lass (Ed.), Speech and Language, Elsevier1982, pp. 271-297.
[18] V. Beachley, G. Ma, C. Papadimitriou, M. Gibson, M. Corvelli, J. Elisseeff, Extracellular matrix particle–glycosaminoglycan composite hydrogels for regenerative medicine applications, Journal of Biomedical Materials Research Part A 106(1) (2018) 147-159.
[19] A.K. Miri, Mechanical Characterization of Vocal Fold Tissue: A Review Study, Journal of Voice 28(6) (2014) 657-667.
Image credit: 1. Smashicons. (nd). Larynx Icons. Retrieved from https://www.flaticon.com/free-icons/larynx. 2. Freepik. (nd). Cell icons. Retrieved from https://www.flaticon.com/free-icons/cell.
