
MRM Insights: Transcriptional Rejuvenation of Aged Muscle Stem Cells

Darren Blackburn

Vahab Soleimani
Every month, in the MRM Insights, a member of the MRM Network writes about stem cells and regenerative medicine from a different perspective. This month, Dr. Vahab Soleimani, Associate Professor at the Department of Human Genetics at McGill and investigator at the Lady Davis Institute for Medical Research, and Darren Blackburn, Ph.D Candidate in his lab, discuss the transcriptional rejuvenation of aged muscle stem cells.
Transcriptional Rejuvenation of Aged Muscle Stem Cells by a Young Niche Environment
Healthy skeletal muscle has a potent regenerative capacity owing to the presence of a small population of muscle stem cells (MuSCs) which ensure the lifelong maintenance and repair of the tissue. MuSCs, nor any other cell within the body, do not exist in a vacuum; their health and function are dictated not only by their intrinsic properties but also by the interactions they have with their niche environment. The niche environment in which MuSCs reside plays a critical role in their function. The niche encompasses numerous non-myogenic cells such as Fibro-Adipogenic Progenitors (FAPs), macrophages, endothelial cells, and fibroblasts amongst others, which have been shown to be important in regulating MuSC quiescence, proliferation, and differentiation1-4. In addition to resident cells, the niche environment is also affected by systemic factors originating from blood circulation as well as structural components such as myofibers and the composition of the extracellular matrix (ECM)5-8.
Aging has a dramatic negative effect on skeletal muscle and consequently on the MuSC’s niche environment because of increased inflammation, fibrosis, fat infiltration, and muscle atrophy during advanced age. These alterations in the muscle perturb the crosstalk between MuSCs and neighboring cells, leading to a breakdown in MuSC support from the niche9-11. Historically, parabiosis experiments have shown that exposure of aged muscle to young circulatory factors can restore the regenerative capacity of aged muscle12, demonstrating the effect that extrinsic factors can have on MuSC function.
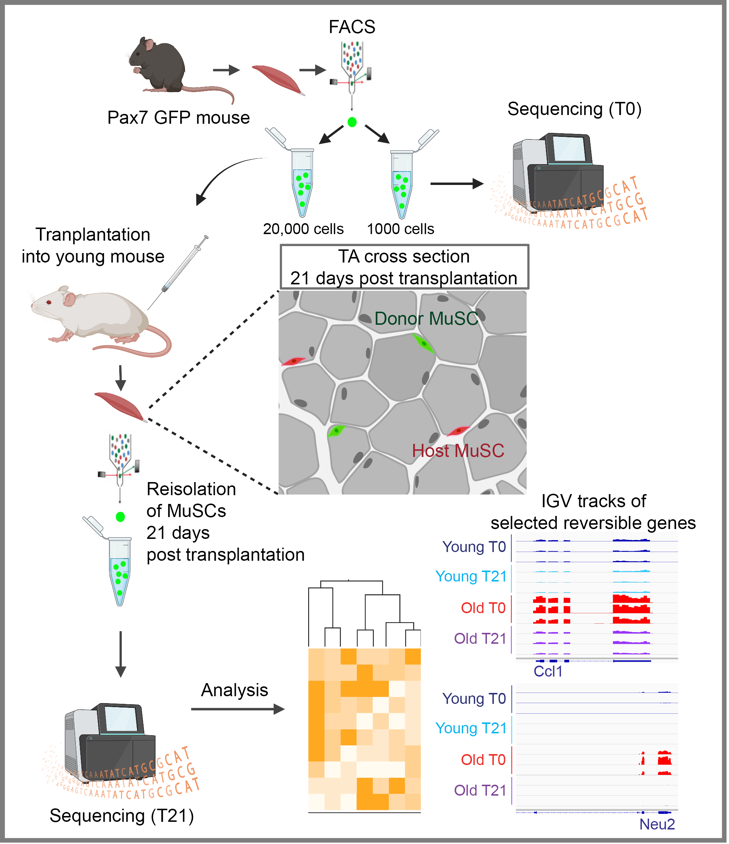
Figure 1: Diagram of the transplantation model used in the study. Created with Biorender.com
In a recent study, we took a multiprong approach using single-cell genomics and analyzed the effect that aging has on the population dynamics, the epigenome, and the transcriptome of MuSCs13. Analysis of scRNA-seq data showed that age-related muscle stem cell loss is not random, and that specific subpopulations are preferentially lost in aged mice. Interestingly, subpopulations that are retained in aged mice increase the expression of stress response and antioxidant genes. This finding suggests that activation of antioxidant and stress response genes may allow these cells to survive in the aged muscle environment.
To determine if age-altered gene expression is reversible, we designed an in vivo muscle stem cell transplantation model in which we isolated GFP-labeled MuSCs from old donors and transplanted them into the skeletal muscle of young host mice13. The engrafted MuSCs were given three weeks to home to the niche and return to quiescence. The engrafted cells were reisolated and had their whole transcriptome analyzed by RNA-seq. Strikingly, approximately half of all age-altered genes were restored (transcriptionally rejuvenated) as a result of exposure to a young niche environment. The reversibility of the MuSC transcriptome suggests that targeting the niche environment can be an important future therapeutic venue to restore stem cell-mediated endogenous muscle repair in aging and other muscle-wasting conditions.
Although this study provides critical new insight into the role of niche environment in the regulation of MuSC gene expression, many fundamental questions remain unanswered. For example, which factors within the young niche environment act as rejuvenating elements? Furthermore, aging also leads to a significant alteration in chromatin accessibility and DNA methylation; can the young niche epigenetically rejuvenate aged MuSCs? In other words, can age-related alterations in chromatin be restored by niche exposure? Furthermore, extending this analysis to non-myogenic cells within the skeletal muscle to study their potential transcriptional rejuvenation by young niche environment can identify common factors or biological pathways which can be used as molecular targets to enhance muscle regeneration.
To learn more about this topic, see the recent paper published by the Soleimani Lab and their collaborators in Nature Comms: Transcriptional reprogramming of skeletal muscle stem cells by the niche environment.
References
1 Biferali, B., Proietti, D., Mozzetta, C. & Madaro, L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Frontiers in Physiology 10 (2019). https://doi.org:10.3389/fphys.2019.01074
2 Joe, A. W. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153-163 (2010). https://doi.org:10.1038/ncb2015
3 Verma, M. et al. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 23, 530-543 e539 (2018). https://doi.org:10.1016/j.stem.2018.09.007
4 Ratnayake, D. et al. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature 591, 281-287 (2021). https://doi.org:10.1038/s41586-021-03199-7
5 Li, H. et al. Muscle-secreted granulocyte colony-stimulating factor functions as metabolic niche factor ameliorating loss of muscle stem cells in aged mice. The EMBO Journal 38, e102154 (2019). https://doi.org:10.15252/embj.2019102154
6 Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078-1081 (2010). https://doi.org:10.1126/science.1191035
7 Garg, K. & Boppart, M. D. Influence of exercise and aging on extracellular matrix composition in the skeletal muscle stem cell niche. J Appl Physiol (1985) 121, 1053-1058 (2016). https://doi.org:10.1152/japplphysiol.00594.2016
8 Oh, J. et al. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging (Albany NY) 8, 2871-2896 (2016). https://doi.org:10.18632/aging.101098
9 Ancel, S., Mashinchian, O. & Feige, J. N. Adipogenic progenitors keep muscle stem cells young. Aging 11, 7331-7333 (2019). https://doi.org:10.18632/aging.102304
10 Lukjanenko, L. et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 24, 433-446.e437 (2019). https://doi.org:https://doi.org/10.1016/j.stem.2018.12.014
11 Lukjanenko, L. et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med 22, 897-905 (2016). https://doi.org:10.1038/nm.4126
12 Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760-764 (2005). https://doi.org:10.1038/nature03260
13 Lazure, F. et al. Transcriptional reprogramming of skeletal muscle stem cells by the niche environment. Nat Commun 14, 535 (2023). https://doi.org:10.1038/s41467-023-36265-x
