MRM Insights: How Postnatal Neurogenesis Modifies Hippocampal Function

Wei-Hsiang Huang

Max Kowalczyk
Every month, in the MRM Insights, a member of the MRM Network offers a unique perspective on the topics of stem cells and regenerative medicine. In this edition, Dr. Wei-Hsiang Huang, who serves as an Assistant Professor in the Department of Neurology & Neurosurgery at the Center for Research in Neuroscience (CRN), collaborates with Max Kowalczyk, a Ph.D. student in his lab. Together, they delve into the exploration of how postnatal neurogenesis modifies hippocampal function in health and disease.
Understanding How Postnatal Neurogenesis Modifies Hippocampal Function in Health and Disease
Postnatal neurogenesis is an integral component of neural ontogeny and plasticity characterized by the ongoing generation of novel neurons from neural stem cells throughout an organism’s lifespan. Within mammals, postnatal neurogenesis has been documented to occur within several brain regions, notably within the hippocampus and specifically within the subventricular zone of the dentate gyrus [1,2]. The addition of newly generated adult-born dentate granule cells (dGCs) provides structural and functional plasticity to the hippocampal tri-synaptic circuit via the connective features of immature adult-born dGCs [3,4]. Since its discovery and affirmation, adult hippocampal neurogenesis has been implicated within multiple physiological roles, including cognitive processes such as learning and memory and cognitive flexibility. Diseases contributing to the dysregulation of this niche group of stem cells have been associated with neurological disorders (such as clinical dementia, schizophrenia, depression, and bipolar disorders), cognitive impairments, and temporal lobe epilepsy [5-7]. Importantly, many of these diseases are associated with the hyperactivity of the mammalian target of the rapamycin (mTOR) pathway, a cellular signalling cascade that mediates the process of neural growth and development [8,9].
Hyperactivation of the mTOR pathway is a feature of Tuberous Sclerosis Complex (TSC), a multisystemic developmental disorder caused by mutations in the TSC1 or TSC2 genes. While there is a large overlap in the clinical phenotypes resulting from TSC1 and TSC2 mutations, evidence suggests that TSC2 mutations are more frequently detected and elicit more severe neurological phenotypes. Of the hallmark pathologies of TSC, including cortical tubers and non-malignant hamartomas, the most debilitating aspects are those linked to neurological disability [10]. Nearly 90% of patients with TSC develop epilepsy as early as infancy, with many cases becoming intractable [11]. With this, nearly 50% of patients suffer from intellectual disability, cognitive impairments, and autism spectrum disorders (ASD) [8]. TSC is classified as a “mTORopathy” as it impacts the mTOR pathway that plays a critical role in cell growth and metabolism in response to growth factors, stress, energy, and nutrients [12]. mTOR is also a central component of positive feedback loops, which promote network activity by repressing inhibitory synapses onto excitatory neurons [12]. While excessive mTOR activity in postnatally generated dGCs contributes to epileptogenesis, the ability of Tsc-mediated mTOR hyperactivity to regulate seizure susceptibility and elicit cognitive dysfunction remains unknown [13-15].
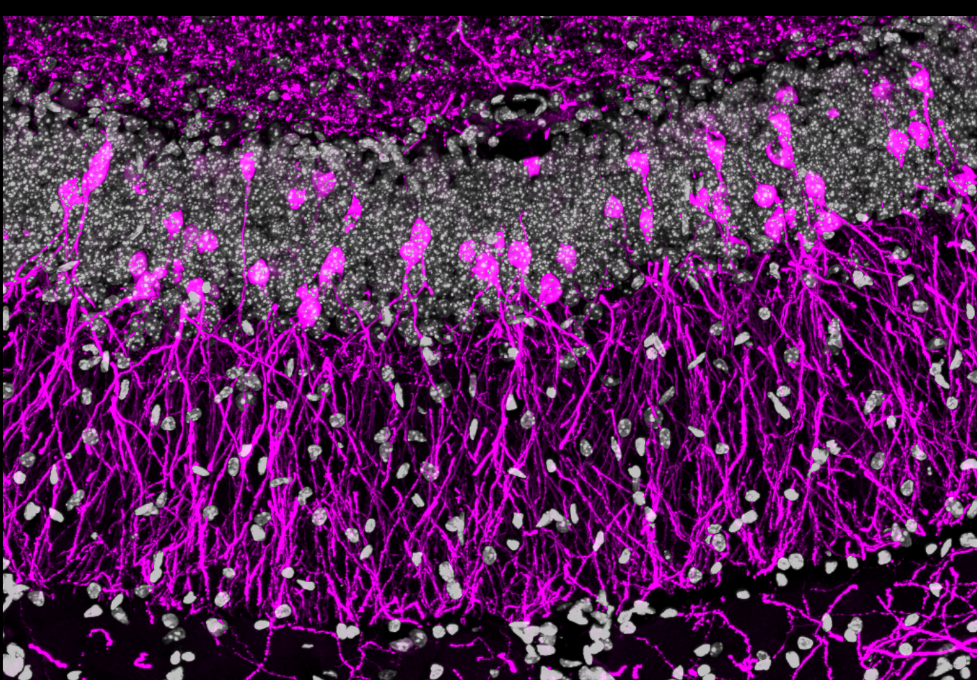
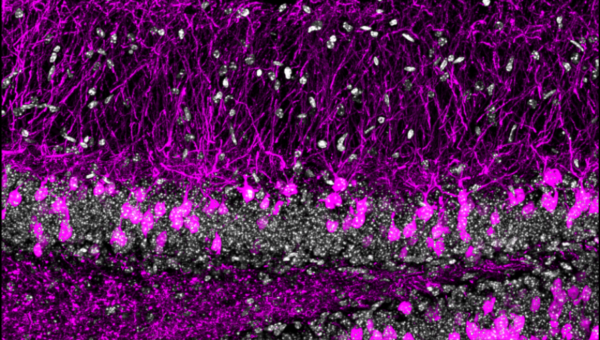
Figure 1: Immunostaining showing hippocampal sections stained with DAPI (grey) as well as reporter mouse line fluorescently labelled adult-born dGCs (magenta).
Rodent models for mTOR pathway activity showed that broadly deleting Tsc1/2 in either glial or neuronal populations show neurocognitive phenotypes. In contrast, it has been shown that mTOR hyperactivity within hippocampal cells can elicit epilepsy [16]. Although these studies outline the ability for pan-neural or pan-glial Tsc loss to generate neurocognitive effects and for mTOR hyperactivity to cause hippocampal dGC abnormalities, the impact of Tsc2 on postnatal dGC neurogenesis remains unknown. To assess the role of Tsc2 on adult-born dGC structure and function, we use a temporally controlled conditional knockout approach to specifically delete Tsc2 in adult-born dGCs and uncover how mTOR activity during postnatal neural stem cell development affects the function of existing healthy networks. We then examine the role of Tsc2 in adult-born dGCs’ cellular growth and morphology and evaluate how a Tsc2 conditional knockout affects cognition and seizure susceptibility in rodent models. We are also exploring the influence of the key mTOR complexes (mTORC1 and mTORC2) on molecular signalling and see if either can be manipulated to rebalance mTOR signalling. This will also allow us to explore a genetic therapeutic approach to potentially regulate Tsc2-induced mTOR hyperactivity, which is more targeted as current therapeutic options rely on the administration of Rapamycin, which is ineffective at treating many cognitive phenotypes and elicits many off-target effects [10,17].
Our current work suggests that Tsc2 expression within adult-born dGCs is essential for regulating neuronal signalling, including mTOR activity and baseline network activity. We also observe that Tsc2 expression within adult-born dGCs is important for various behavioural aspects, including cognition, learning and memory, and seizure susceptibility. With our ongoing research, this targeted approach will allow us to gain insight into understanding the role of Tsc2 in influencing neural cell signalling, morphology, and behaviour within the hippocampus. It will also uncover how Tsc2 signalling within adult-born dGCs may result in various phenotypes observed within patients with TSC and determine how much these adult stem cells contribute to TSC-like symptoms by influencing the function of existing neural networks.
References
1. Triarhou, L. C., & Manto, M. (2021). Postnatal neurogenesis beyond rodents: The groundbreaking research of Joseph Altman and Gopal Das. The Cerebellum, 21(1), 1–8.
2. Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A.-M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4(11), 1313–1317.
3. Toda, T., Parylak, S. L., Linker, S. B., & Gage, F. H. (2018). The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry, 24(1), 67–87.
4. Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., Boström, E., Westerlund, I., Vial, C., Buchholz, B. A., Possnert, G., Mash, D. C., Druid, H., & Frisén, J. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6), 1219–1227.
5. Burghardt, N. S., Park, E. H., Hen, R., & Fenton, A. A. (2012). Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus, 22(9), 1795–1808.
6. Hatami, M., Conrad, S., Naghsh, P., Alvarez-Bolado, G., & Skutella, T. (2018). Cell-biological requirements for the generation of dentate gyrus granule neurons. Frontiers in Cellular Neuroscience, 12.
7. Hattiangady, B., Rao, M., & Shetty, A. (2004). Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiology of Disease, 17(3), 473–490.
8. LaSarge, C. L., & Danzer, S. C. (2014). Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Frontiers in Molecular Neuroscience, 7.
9. Mizuguchi, M., Ohsawa, M., Kashii, H., & Sato, A. (2021). Brain symptoms of tuberous sclerosis complex: Pathogenesis and treatment. International Journal of Molecular Sciences, 22(13), 6677.
10. Karalis, V., Caval-Holme, F., & Bateup, H. S. (2021). Raptor Downregulation Rescues Neuronal Phenotypes in Mouse Models of Tuberous Sclerosis Complex.
11. Blair, J. D., Hockemeyer, D., & Bateup, H. S. (2018). Genetically engineered human cortical spheroid models of tuberous sclerosis. Nature Medicine, 24(10), 1568–1578.
12. Bateup, H. S., Johnson, C. A., Denefrio, C. L., Saulnier, J. L., Kornacker, K., & Sabatini, B. L. (2013). Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron, 78(3), 510–522.
13. Arafa, S. R., LaSarge, C. L., Pun, R. Y. K., Khademi, S., & Danzer, S. C. (2019). Self-reinforcing effects of mtor hyperactive neurons on dendritic growth. Experimental Neurology, 311, 125–134.
14. LaSarge, C. L., Santos, V. R., & Danzer, S. C. (2015). PTEN deletion from adult-generated dentate granule cells disrupts granule cell mossy fiber axon structure. Neurobiology of Disease, 75, 142–150.
15. Pun, R. Y. K., Rolle, I. J., LaSarge, C. L., Hosford, B. E., Rosen, J. M., Uhl, J. D., Schmeltzer, S. N., Faulkner, C., Bronson, S. L., Murphy, B. L., Richards, D. A., Holland, K. D., & Danzer, S. C. (2012). Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron, 75(6), 1022–1034.
16. Ehninger, D., de Vries, P. J., & Silva, A. J. (2009). From mtor to cognition: Molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. Journal of Intellectual Disability Research, 53(10), 838–851.
17. Kaplan, B., Qazi, Y., & Wellen, J. R. (2014). Strategies for the management of adverse events associated with mTOR inhibitors. Transplantation Reviews, 28(3), 126–133.
