MRM Insights: Multipotent Mesenchymal Stromal Cells – Therapeutic Clinical Trials at McGill

Dr. Marie Hudson

Dr. Dominique Farge

Prof. James Martin

Dr. Inés Colmegna
Every month, in the MRM Insights, a member of the MRM Network offers a unique perspective on the topics of stem cells and regenerative medicine. In this edition, Dr. Inés Colmegna, Associate Professor in Rheumatology at the RI-MUHC has partnered with Professor James Martin, Dr. Dominique Farge and Dr. Marie Hudson to discuss the therapeutic clinical trials utilizing mesenchymal stromal cells that are presently underway at McGill.
Multipotent Mesenchymal Stromal Cells: Therapeutic Clinical Trials at McGill
Inés Colmegna1, James Martin2, Dominique Farge3, Marie Hudson4
1 Division of Rheumatology, McGill University Health Centre (MUHC) & Research Institute of the MUHC (RI-MUHC)
2 Respiratory Division, McGill University Health Centre (MUHC) & Meakins-Christie Laboratories, RI-MUHC
3 Department of Internal Medicine, Paris University & Division of Experimental Medicine, McGill University
4 Division of Rheumatology, Jewish General Hospital (JGH) & Lady Davis Institute
Mesenchymal stromal cells (MSCs) are multipotent cells with immunomodulatory, proangiogenic, and anti-fibrotic properties documented in vitro and in animal models. On this premise, MSCs became frequently tested in clinical trials. Most MSC trials are small, early phase, uncontrolled and target a wide range of inflammatory diseases, mainly musculoskeletal, neurological, and cardiovascular conditions. Those trials differ in their design, tissue source (i.e. bone marrow, adipose tissue and umbilical cord), report of product characterization (i.e. cellular identity, purity, potency, viability, functional assays) and handling (i.e. cryopreservation media composition, delivery vehicle, post-thaw time and storage until administration, route of administration, duration of infusion). As a result, although MSC therapies have consistently proven to be safe, the effects of MSC in clinical trials have been variable and often less beneficial than in preclinical studies. Several recommendations have been made to maximize MSC therapeutic effects and to understand the biology underpinning the potential mechanisms of action of MSC. These include improving the reporting of MSC clinical trials with standardized terminology and nomenclature, details on the preparation and analysis of the investigational product including definition of cell sourcing and manufacture (i.e. standardization of the cell-based product from expansion through delivery), objective characterization of cellular populations administered to patients, and well defined study inclusion criteria. The adequate and transparent disclosure of these features are expected to result in reproducible clinical studies and improve the overall credibility of the cell therapy field. Following these recommendations, during the past 2 years, the first two clinical trials using MSC at McGill started recruitment. Both studies are investigator-initiated, used umbilical cord derived MSCs and were designed and initiated in collaboration with clinician and statistical colleagues from the center of reference for stem cell therapy, Paris Cite MATHEC at St-Louis Hospital, Paris-Cité University.
ProTrans19+ (NCT04869397) used MSCs for the treatment of respiratory complications associated with COVID-19. This study tested the hypothesis that the anti-inflammatory, immunomodulatory and pro-reparative effects of allogeneic Wharton’s Jelly (WJ)-MSCs would be safe and improve the clinical status of adults with respiratory complications associated with COVID-19 infection. ProTrans19+ was a randomized phase II, double-blind, placebo-controlled clinical trial with the objective of evaluating the efficacy of a single intravenous infusion of a fixed dose of ProTrans® (100 million MSCs per patient) in the treatment of “severe” COVID-19 pneumonia, defined as patients who did not achieve an oxygen saturation > 96% on 4 L/min of O2 and who were not on ‘non-invasive’ ventilation, invasive mechanical ventilation or ECMO. All participants received what was considered standard of care treatment for COVID at their time of enrolment (e.g. dexamethasone, anti-viral therapy, anti-cytokine therapy). In addition, patients in the active arm received a single intravenous infusion of allogeneic MSC(WJ) (ProTrans®). ProTrans® contained pooled ex vivo expanded MSCs from 5 donors at passage 3. The product was provided cryopreserved by NextCell Pharma, and the cryobags were thawed at bedside prior to administration. The primary outcome of the study was a composite end point defined by the rate of use of mechanical ventilation (i.e., need for intubation) or death at 15 days after inclusion. ProTrans19+ tested a number of secondary endpoints (i.e., clinical evolution, survival, time to clinical improvement, duration of hospitalization and ICU stay) as well as exploratory mechanistic analyses. The study recruited nineteen patients at two sites (i.e., MUHC-Glen site and JGH). ProTrans19+ was terminated prematurely due to futility. The analysis of the study is ongoing.
CARE-SSc (NCT04356287) is a single center study (JGH) using MSCs for the treatment of patients with severe systemic sclerosis (SSc or scleroderma). This study is testing the hypothesis that a single or two infusions 3 months apart of MSC(WJ) are safe and efficacious for the treatment of adults with severe SSc who in addition meet two other criteria: (1) inadequate response or adverse events necessitating discontinuation of standard therapy [usually consisting of methotrexate 25 mg subcutaneously (or as tolerated) per week and/or mycophenolate mofetil 2-3 gm/d (or as tolerated) for at least 3 months], and (2) ineligible or refusal to undergo autologous hematopoietic stem cell transplant. CARE-SSc is a randomized phase I/II, double-blind, placebo-controlled trial. It proposes to enroll 18 SSc patients in 3 successive blocks of 6 patients each. Participants are randomized to one of two treatment arms or a placebo arm (total of 6 patients per arm). Within each block, the 6 patients are randomized in a 2:2:2 ratio in one of the following arms: placebo, 1 infusion of MSCs (month 0), or 2 infusions of MSCs (months 0 and 3). Second infusions of MSCs are performed only in the absence of Treatment Related Severe Adverse Events (TRSAE). Randomization into blocks 2 and 3 are staggered, to allow the detection of TRSAE prior to inclusion of patients in a subsequent block, i.e. the second block will be randomized only in the absence of TRSAE one month after the first infusion of all 6 patients in block one, and similarly for the third block. Each infusion of active product consists of 1 million MSC/kg. CARE-SSc primary objective is safety assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 classification. The primary outcome is TRSAE defined as a grade ≥ 3 CTCAE treatment-related adverse event assessed at one month after each infusion. The secondary objective of this study is to seek evidence for a possible efficacy signal to inform future phases of this clinical research program. The main efficacy outcome is the modified Rodnan skin score (mRSS, validated measure of skin fibrosis), computed as the difference between month 12 (M12) and M0. In addition to efficacy, other secondary (e.g, quality of life, mortality, etc), and mechanistic exploratory outcomes will be reported. The product is provided cryopreserved by the Center for Cellular Therapy at the Medical University of South Carolina, and the cryobags are thawed at bedside prior to administration. CARE-SSc recruitment is ongoing, the enrollment of the first block of 6 patients was completed. The Data Safety and Monitoring Board (DSMB) has approved the start of recruitment for the second block.
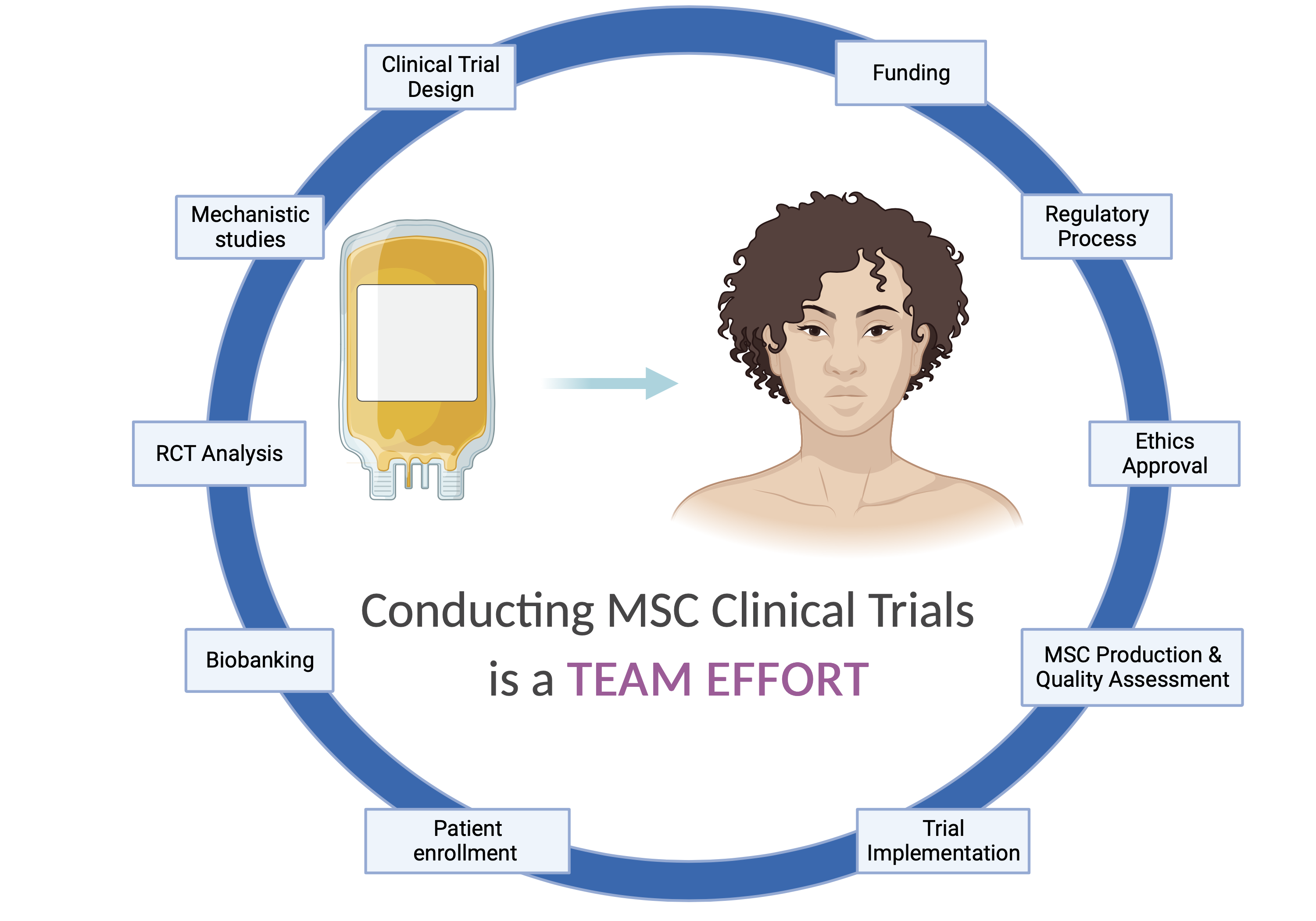
These two MSC trials, unprecedented at McGill, allow us now to reflect on two points: feasibility / requirements for the implementation of MSC clinical trials and next steps. ProTrans19+ and CARE-SSc are a proof of concept for the feasibility of conducting MSC clinical trials at McGill. ProTrans19+ was funded by the MUHC-Foundation and the Lady Davis Institute with the in-kind contribution of MSC products by NextCell Pharma. CARE-SSc was funded by an operating grant from CIHR (PI: M. Hudson). None of these two trials would have been implemented without the contribution of many colleagues, some of whom have unique expertise in other types of cell therapy trials, who were/are instrumental in the design, implementation, and analysis of ProTrans19+ and CARE-SSc. Big credit should go to the studies’ DSMBs, led by Dr Jean-Pierre Routy (ProTrans19+) and Dr Jacques Galipeau (CARE-SSc). Conducting cell therapy trials truly requires a team effort and can’t be done without having access to unique facilities such as the Cellular Therapy Laboratory – CTL at the MUHC, the Clinical Research Unit at the JGH and international collaborations. The academic and collaborative environment at McGill together with its existing expertise, facilities and trust of participants allowed conducting MSC clinical trials.
It is well accepted that inconsistent outcomes from MSC trials, related to underlying patient biology or procedural differences at trial sites, made it challenging to replicate and conclude on the therapeutic benefits of MSCs. It was recently suggested that patient stratification metrics paired with the selection of therapeutically potent MSCs (using rigorous critical quality attributes and/or engineered MSC products) represent a path forward to improve clinical successes and regulatory endorsements. We expect that regardless of their outcome, ProTrans19+ and CARE-SSc will teach us mechanistic insights that will allow the optimization of MSC products and improve the selection of patients who may benefit from them. Moreover, these trials provide opportunities to pursue basic research into mechanisms of MSC effects and biomarkers of treatment response. They will also inform the design of future, more advanced clinical trials to test efficacy.
References
1. Wilson, A.J., Rand, E., Webster, A.J. et al. Characterisation of mesenchymal stromal cells in clinical trial reports: analysis of published descriptors. Stem Cell Res Ther 12, 360 (2021).
2. Wiese D.M., Wood C.A., Braid L.R. From Vial to Vein: Crucial Gaps in Mesenchymal Stromal Cell Clinical Trial Reporting. Front Cell Dev Biol 10, 867426 (2022).
3. Galipeau J., Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 22, 824 (2018).
4. Robb K.P., Galipeau J., Shi Y., Schuster M., Martin I., Viswanathan S. Failure to launch commercially-approved mesenchymal stromal cell therapies: what’s the path forward? Proceedings of the International Society for Cell & Gene Therapy (ISCT) Annual Meeting Roundtable held in May 2023, Palais des Congrès de Paris, Organized by the ISCT MSC Scientific Committee. Cytotherapy:S1465-3249(23)01049-6 (2023).
